Abstract
Purpose
Commercial enzyme-linked immunosorbent assay (ELISA) kits have been considered less reliable for children than for adults. The aim of this study was to compare four ELISA kits and in-house immunoblotting based on the analysis of anti-H. pylori-IgG antibody reactivity.
Methods
A total of 399 serum samples were collected at the GNU Hospital during 1998-1999. All sera were tested using ELISA and immunoblotting. Statistically significant differences were determined by the χ2 test.
Results
The overall seropositivity rates using GAP IgG, Genedia IgG, HM-CAP, Pyloriset EIA-G, and immunoblotting were 13.0%, 25.1%, 18.3%, 15.8%, and 62.9%, respectively. Immunoblotting showed a higher seropositivity rate than did all four ELISA kits in all age groups. Genedia IgG had the highest seropositivity among the ELISA kits. The seropositivity rate for children aged 13 to 18 months was lowest, and that of children aged 15 years was highest (90.0%). The seropositivity rate for children aged 7 months to 5 years was significantly lower than that for children aged 6 to 15 years among the four ELISA kits (p<0.0001) and immunoblotting (p=0.02).
Conclusion
Immunoblotting is the most sensitive test for detection of anti-Helicobacter pylori IgG antibodies among the serological tests in this study. These results emphasize the need for standardization when commercial ELISA tests are used in different nations or in young age groups. Immunoblotting could be a suitable noninvasive assay for serodiagnosis and seroepidemiologic study of H. pylori infection in Korean children.
Diagnostic tests for Helicobacter pylori infection can be divided into two categories in terms of method: invasive or noninvasive [1-3]. The invasive methods, such as culture, histopathologic examination, and urease tests, require endoscopic biopsies. Noninvasive tests include the urea breath test and serologic tests. Serologic testing is an easy, noninvasive, and commercially available method to detect anti-H. pylori IgG. Commercial enzyme-linked immunosorbent assay (ELISA) kits could be advantageous in screening children for anti-H. pylori IgG. Helicobacter strains that prevail in Asia may exhibit antigenic properties that differ from those of Western countries where the ELISA kits are developed [4]. The ELISA for anti-H. pylori IgG in children showed controversial results with various sensitivities and specificities [5,6], and serological testing was considered to be a less reliable test for children than for adults in Europe [7,8]. The immunoblot assay has been an alternative serologic assay available to diagnose H. pylori infection in children [9].
The aim of this study was to compare the diagnostic values of four commercial ELISA kits and an in-house immunoblot assay in children in Jinju city, South Korea.
A total of 399 serum samples from patients without gastrointestinal disease were collected at the Gyeongsang National University Hospital (GNUH) from 1998 to 1999 and stored at -20℃ until analysis. Patient age ranged from 0 to 15 years, and the patients were divided into 20 groups according to age (Table 1). All sera were provided by the GNUH, a member of the National Biobank of Korea, after the permission from the hospital ethics committee (GNUHIRB-2012-003).
Four commercial ELISA tests were chosen in this study to detect serum IgG against H. pylori: GAP IgG (Bio-Rad, Hercules, CA, USA) against H. pylori outer-membrane antigen, Genedia IgG (MBRIHP-2, Green Cross Co., Seoul, South Korea) using whole-cell sonicates of H. pylori strains isolated from Korean patients with chronic gastritis, HM-CAP (Enteric Products, Stony Brook, NY, USA) against high-molecular-weight cell-associated proteins of H. pylori, and Pyloriset EIA-G (Orion Diagnostica, Espoo, Finland) against acid glycine-extracted surface antigens of H. pylori. All tests were performed as a single batch according to the manufacturers' instructions. The optical density of each sample was translated into an ELISA value as suggested. A positive test was obtained when the ELISA value exceeded a specific level as defined by the manufacturers (GAP IgG=20 antibody titer; Genedia IgG=mean negative control's OD+0.4; HM-CAP=2.2; Pyloriset EIA-G=300).
Helicobacter pylori strain 51 isolated from patients at GNUH was cultivated overnight at 38℃ in 10% CO2 and a 100% humidity atmosphere. For preparation of whole-cell proteins, cells were washed with a washing solution (0.1 M phosphate buffer solution [PBS], pH 7.4) and resuspended with PBS, and phenylmethylsulfonyl fluoride (PMSF) was then added. The cells were disrupted with an ultrasonicator (Ultrasonics W-380) to a protein concentration of 20 µg/mL by the Lowry method.
The whole-cell lysate of H. pylori strain 51 was separated by 10%- to 20%-gradient SDS-PAGE and then transferred onto a nitrocellulose filter. The blot was incubated with a 1:5 dilution of serum and subsequently labeled with alkaline phosphatase-conjugated goat anti-human IgG (Promega) before staining with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate. The presence of a 120,000-MW protein (120-kDa antigen [CagA]) band was considered seropositive [10].
Responses to antigens in the immunoblot results were divided into three patterns (Fig. 1). Pattern I was defined as CagA antigen positive. Pattern II was defined as antigen positive, but without CagA antigen. Pattern III was defined as no positive reactivity. A serum sample was considered to have tested positive by immunoblot analysis if it reacted with the 120-kDa band (Pattern I).
The data were analyzed using PASW Statistics 18. Statistically significant differences in the seropositivity rate among age groups were determined using the χ2 test. Nonparametric correlation (Spearman's rho test) was used to analyze the correlations among the seropositivity rates of the four ELISA kits and immunoblotting. p-values <0.05 were considered to be statistically significant.
Fig. 2 shows the seropositivity rates of anti-H. pylori IgG of the four commercial ELISA kits and immunoblotting according to the age. The overall seropositivity rates of GAP IgG, Genedia IgG, HM-CAP, Pyloriset EIA-G, and immunoblotting were 13.0%, 25.1%, 18.3%, 15.8%, and 62.9%, respectively. The seropositivity rates for immunoblotting were higher than those for the four ELISA kits in all age groups. The overall seropositivity rate for Genedia IgG was the highest among all ELISA kits. The seropositivity rate in the 13- to 18-month-old group was lowest among all age groups (Fig. 2).
The seropositivity rate increased with age and was highest in the 15-year-old group (90.0% on immunoblotting) (Fig. 2). There was no statistical difference between males and females according to ELISA and immunoblotting (Table 2). The seropositivity rate of children aged 7 months to 5 years was significantly lower than that of children aged 6 to 15 years for the four ELISA kits (p<0.0001) and immunoblotting (p=0.02) (Fig. 3).
In 0- to 3-month-old infants, the seropositivity rate for GAP IgG, Genedia IgG, HM-CAP, Pyloriset EIA-G, and immunoblotting was 10%, 40%, 10%, 10%, and 80%, respectively. In 4- to 6-month-old infants, the rate was 5%, 45%, 5%, 5%, and 85%, respectively. The discrepancy in seropositivity rates between the ELISA kits and immunoblotting was highest in these two age groups. The high seropositivity rates of immunoblotting in these age groups might be related to the level of maternal anti-H. pylori IgG transported to the fetus via the placenta.
The seropositivity rate for Genedia IgG and Pyloriset EIA-G was moderately correlated with that for immunoblotting (Genedia IgG, R=0.396; Pyloriset EIA-G, R=0.318; both p<0.0001). The seropositivity rates for GAP IgG and HM-CAP were also mildly correlated with those for immunoblotting (GAP IgG, R=0.159; HM-CAP, R=0.229; both p<0.0001). Immunoblotting results were divided into the three patterns previously described: Pattern I comprised 62.9%, Pattern II comprised 33.3%, and Pattern III comprised 3.8% of immunoblotting results. Only Pattern I was considered positive. In cases of seropositivity using GAP IgG, Genedia IgG, HM-CAP, and Pyloriset EIA-G, Pattern I comprised 82.7%, 96.0%, 86.3%, and 98.4% of immunoblotting results, respectively (Fig. 4), and pattern II comprised 17.3%, 4.0%, 13.7%, and 1.6%, respectively. No results showed Pattern III.
The present comparison of four commercially available ELISA kits and in-house immunoblotting analysis for the serodiagnosis of H. pylori infection in 399 Korean children without gastrointestinal diseases revealed a large discrepancy in seropositivity rates between the ELISA kits and immunoblotting. Determination of antibody levels is affected by the antigen preparations used in the assay. However, most commercial assays are generally limited by the use of antigens that are sensitive and specific only for populations in which the assays were validated [11]. Despite the high accuracy reported in Western countries, the commercial serological tests in some studies were unsatisfactory when used in Chinese [12] and Korean people [13,14]. H. pylori antigen obtained from Korean H. pylori strains was used in the Genedia kit. The seropositivity rate of Genedia IgG was higher than that of the other three ELISA kits. In 1998, the seroprevalence of H. pylori infection in asymptomatic Korean children (n=2,336) was 17.2% using this ELISA test [15]. The difference between our results and those of previous studies might be related to the sensitivity and specificity of the Genedia IgG ELISA (80% and 84.8% in children) [16].
The accuracy of ELISA is lower in children than in adults because of the differences in the immune response [17]. Immunoblotting was more sensitive (100%) and specific (88%) than ELISA in the evaluation of H. pylori infection in children [9]. In the present study, the accuracy of commercial assays was also greatly reduced when serum from younger patients, especially those younger than 5 years, was evaluated.
Most strains of H. pylori isolated in Korea and Japan are CagA-positive strains; we considered positive as immunoreactivity against a 120-kDa band (immunoblot Pattern I) [18]. The diagnostic sensitivity and specificity of commercially available immunoblot assays indicated that they were accurate, but evaluation of CagA status and VacA was poor [19]. The CagA-positive rate in our study was 62.9% by in-house immunoblot analysis, and this result was higher compared with the CagA-positivity rate in French children (43.1%) by the immunoblot technique [9]. More than 80% of seropositive ELISA results were CagA positive in our study. This is consistent with the finding that 94% of Korean children with recurrent abdominal pain and H. pylori gastritis had a cagA-positive genotype [20].
In the present study, Pattern II showed that many antigens without CagA antigen were immunoreactive. The proportion of Pattern II was 33.3% in this study. Pattern II involved immunoreactive antigens other than CagA. This result might be related to the fact that the immunoreactive rates of the 25-, 30-, and 19.5-kDa antigens were higher than that of the 120-kDa antigen in children [6]. Further study is needed to clarify the geographical differences in immune reactions according to age and strain.
There were several limitations to the present study, including the fact that the study population comprised asymptomatic children, no endoscopic study was performed, the specificity and sensitivity of in-house immunoblotting was not proven, the study population was small in each age group, and Pattern II may be positive in other studies [9]. The CagA-positive H. pylori strain was predominant in Korea, and we considered that only Pattern I was positive. Positivity of antibody against H. pylori could not differentiate the recent and past infections and not suitable for follow-up tests after chemotherapy of H. pylori [21,22].
In summary, the seropositivity rate for in-house immunoblotting was higher than that for commercially available ELISA kits. The seropositivity rate of children 7 months to 5 years of age was lower than that of children 6 to 15 years of age.
In conclusion, in-house immunoblotting is the most sensitive test with which to detect anti-H. pylori IgG antibodies among the serological tests in this study. These results emphasize the need for standardization when commercial ELISA tests are used in different nations or in young age groups. In-house immunoblotting could be a suitable noninvasive assay for serodiagnosis and seroepidemiologic study of H. pylori infection in Korean children.
Figures and Tables
Fig. 1
Immunoblot assay results were classified into three patterns based on immunoreactive bands. Only Pattern I, which shows reactivities against 120-kDa antigens as well as other antigens of H. pylori, was considered to be a specific marker of H. pylori infection in this study. Panel A shows a Ponceau S-stained nitrocellulose membrane onto which marker proteins and separated H. pylori antigen were transferred.
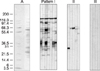
Fig. 2
Seropositivity rates of the four commercial ELISA kits and immunoblotting according to age. The seropositivity rates increased with age. The seropositivity rates of immunoblotting were higher than those of the ELISA kits, and the discrepancy in the seropositivity rates of anti-H. pylori IgG antibody was highest in 0- to 6-month-old infants.

Fig. 3
Comparisons of seropositivity rates for GAP IgG, Genedia IgG, HM-CAP, Pyloriset EIA-G, and immunoblotting in children 7 months to 5 years of age and 6 to 15 years of age. The seropositivity rates in children aged 6 years and older were higher than those of children aged 7 months to 5 years of age in all ELISA kits and immunoblotting. *p<0.0001, **p=0.02.
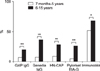
Fig. 4
Proportion of immunoblot patterns in the seropositive cases of the four ELISA kits. More than 80% were Pattern I. There were no results showing Pattern III.

ACKNOWLEDGEMENTS
This study was funded by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0820050). The biospecimens for this study were provided by Gyeongsang National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health & Welfare.
References
1. Brown KE, Peura DA. Diagnosis of Helicobacter pylori infection. Gastroenterol Clin North Am. 1993. 22:105–115.
2. Carpenter HA, Talley NJ. Gastroscopy is incomplete without biopsy: clinical relevance of distinguishing gastropathy from gastritis. Gastroenterology. 1995. 108:917–924.

3. Cutler AF, Havstead S, Ma CK, Blaser MJ, Perez-Perez GI, Schubert TT. Accuracy of invasive and non-invasive test to diagnose Helicobacter pylori infection. Gastroenterology. 1995. 109:136–141.

4. Leung Wk, Chan FKL, Falk MS, Suen R, Sung JJY. Comparison of two rapid whole-blood tests for Helicobacter pylori infection in Chinese patients. J Clin Microbiol. 1998. 36:3441–3442.

5. Khanna B, Cutler A, Israel NR, Perry M, Lastovica A, Fields PI, et al. Use caution with serologic testing for Helicobacter pylori infection in children. J Infect Dis. 1998. 178:460–465.

6. Raymond J, Sauvestre C, Kalach N, Bergeret M, Dupont C. Immunoblotting and serology for diagnosis of Helicobacter pylori infection in children. Pediatr Infect Dis J. 2000. 19:118–121.

7. Drumm B, Koletzko S, Oderda G. Helicobacter pylori infection in children: a consensus statement. J Pediatr Gastroenterol Nutr. 2000. 30:207–213.
8. Kindermann A, Konstantopoulos N, Lehn N, Demmelmair H, Koletzko S. Evaluation of two commercial enzyme immunoassays, testing immunoglobulin G (IgG) and IgA responses, for diagnosis of Helicobacter pylori infection in children. J Clin Microbiol. 2001. 39:3591–3596.

9. Raymond J, Sauvestre C, Kalach N, Bergeret M, Dupont C. Immunoblotting and serology for diagnosis of Helicobacter pylori infection in children. Pediatr Infect Dis J. 2000. 19:118–121.

10. Youn HS, Baik SC, Lee WK, Cho MJ, Ryou HH, Choi HJ, et al. Serodiagnosis of Helicobacter pylori infection. J Bacteriol Virol. 1990. 25:463–474.
11. Best LM, Veldhuyzen van Zanten SJ, Sherman PM, Berganson GS. Serological detection of Helicobacter pylori antibodies in children and their parents. J Clin Microbiol. 1994. 32:1193–1196.

12. Leung WK, Ng EK, Chan FK, Chung SC, Sung JJ. Evaluation of three commercial enzyme-linked immunosorbent assay kits for diagnosis of Helicobacter pylori in Chinese patients. Diagn Microbiol Infect Dis. 1999. 34:13–17.

13. Lee KL, Lim CY, Lee DH, Yoon JH, Lee HJ, Chung HC, et al. Evaluation of CtuickVue H. pylori test and IgG ELISA test in Helicobacter pylori infection. Korean J Gastroenterol. 1997. 29:35–40.
14. Hong SP, Park HJ, Park IS, Lee KW, Kim HG. Serological diagnosis of Helicobacter pylori infection: comparison of diagnostic values between HM-CAP (EPI) test and GAP (Bio-Rad) test. Korean J Gastroenterol. 1995. 27:167–173.
15. Kim JH, Kim HY, Kim NY, Kim SW, Kim JG, Kim JJ, et al. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001. 16:969–975.

16. Jung IS, Kim SW, Ko JS, Kim NY, Kim JG, Kim JH, et al. Accuracy of Genedia™ H. pylori ELISA for the diagnosis of Helicobacter pylori infection in Korean population. Korean J Med. 2001. 61:17–23.
17. Andersen LP, Wewer AV, Christiansen KM, Tvede M, Hansen JP, Henriksen FW, et al. The humoral immune response to Helicobacter pylori infection in children with recurrent abdominal pain. APMIS. 1994. 102:457–464.

18. Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999. 37:2274–2279.

19. Yamaoka Y, Kodama T, Graham DY, Kashima K. Comparison of four serological tests to determine the CagA or VacA status of Helicobacter pylori strains. J Clin Microbiol. 1998. 36:3433–3434.

20. Ko JS, Kim KM, Oh YL, Seo JK. cagA, vacA, and iceA genotypes of Helicobacter pylori in Korean children. Pediatr Int. 2008. 50:628–631.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download