1. Yang XY, Xi MR, Yang KX, Yu H. Prognostic value of estrogen receptor and progesterone receptor status in young Chinese ovarian carcinoma patients. Gynecol Oncol. 2009. 113:99–104.
2. Tangjitgamol S, Manusirivithaya S, Khunnarong J, Jesadapatarakul S, Tanwanich S. Expressions of estrogen and progesterone receptors in epithelial ovarian cancer: a clinicopathologic study. Int J Gynecol Cancer. 2009. 19:620–627.
3. Hogdall EV, Christensen L, Hogdall CK, Blaakaer J, Gayther S, Jacobs IJ, et al. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the 'MALOVA' ovarian cancer study. Oncol Rep. 2007. 18:1051–1059.
4. Lindgren PR, Cajander S, Backstrom T, Gustafsson JA, Makela S, Olofsson JI. Estrogen and progesterone receptors in ovarian epithelial tumors. Mol Cell Endocrinol. 2004. 221:97–104.
5. Arias-Pulido H, Smith HO, Joste NE, Bocklage T, Qualls CR, Chavez A, et al. Estrogen and progesterone receptor status and outcome in epithelial ovarian cancers and low malignant potential tumors. Gynecol Oncol. 2009. 114:480–485.
6. Iversen OE, Skaarland E, Utaaker E. Steroid receptor content in human ovarian tumors: survival of patients with ovarian carcinoma related to steroid receptor content. Gynecol Oncol. 1986. 23:65–76.
7. Geisler JP, Wiemann MC, Miller GA, Geisler HE. Estrogen and progesterone receptor status as prognostic indicators in patients with optimally cytoreduced stage IIIc serous cystadenocarcinoma of the ovary. Gynecol Oncol. 1996. 60:424–427.
8. Lee P, Rosen DG, Zhu C, Silva EG, Liu J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecol Oncol. 2005. 96:671–677.
9. Kommoss F, Pfisterer J, Thome M, Schafer W, Sauerbrei W, Pfleiderer A. Steroid receptors in ovarian carcinoma: immunohistochemical determination may lead to new aspects. Gynecol Oncol. 1992. 47:317–322.
10. Munstedt K, Steen J, Knauf AG, Buch T, von Georgi R, Franke FE. Steroid hormone receptors and long term survival in invasive ovarian cancer. Cancer. 2000. 89:1783–1791.
11. Bizzi A, Codegoni AM, Landoni F, Marelli G, Marsoni S, Spina AM, et al. Steroid receptors in epithelial ovarian carcinoma: relation to clinical parameters and survival. Cancer Res. 1988. 48:6222–6226.
12. Slotman BJ, Nauta JJ, Rao BR. Survival of patients with ovarian cancer. Apart from stage and grade, tumor progesterone receptor content is a prognostic indicator. Cancer. 1990. 66:740–744.
13. Harding M, Cowan S, Hole D, Cassidy L, Kitchener H, Davis J, et al. Estrogen and progesterone receptors in ovarian cancer. Cancer. 1990. 65:486–491.
14. Sevelda P, Denison U, Schemper M, Spona J, Vavra N, Salzer H. Oestrogen and progesterone receptor content as a prognostic factor in advanced epithelial ovarian carcinoma. Br J Obstet Gynaecol. 1990. 97:706–712.
15. Hempling RE, Piver MS, Eltabbakh GH, Recio FO. Progesterone receptor status is a significant prognostic variable of progression-free survival in advanced epithelial ovarian cancer. Am J Clin Oncol. 1998. 21:447–451.
16. Schwartz PE, MacLusky N, Merino MJ, Livolsi VA, Kohorn EI, Eisenfeld A. Are cytosol estrogen and progestin receptors of prognostic significance in the management of epithelial ovarian cancers? Obstet Gynecol. 1986. 68:751–758.
17. Anderl P, Fuith LC, Daxenbichler G, Marth C, Dapunt O. Correlation between steroid hormone receptors, histological and clinical parameters in ovarian carcinoma. Gynecol Obstet Invest. 1988. 25:135–140.
18. Rose PG, Reale FR, Longcope C, Hunter RE. Prognostic significance of estrogen and progesterone receptors in epithelial ovarian cancer. Obstet Gynecol. 1990. 76:258–263.
19. Masood S, Heitmann J, Nuss RC, Benrubi GI. Clinical correlation of hormone receptor status in epithelial ovarian cancer. Gynecol Oncol. 1989. 34:57–60.
20. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000. 70:209–262.
21. Serov SF, Scully RE, Sobin LH. International histological classification and staging of tumors: histologic typing of ovarian tumors. 1973. Geneva: World Health Organization.
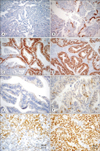
22. Rosen DG, Huang X, Deavers MT, Malpica A, Silva EG, Liu J. Validation of tissue microarray technology in ovarian carcinoma. Mod Pathol. 2004. 17:790–797.
23. Akahira J, Suzuki T, Ito K, Kaneko C, Darnel AD, Moriya T, et al. Differential expression of progesterone receptor isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Jpn J Cancer Res. 2002. 93:807–815.
24. R: Development Core Team. R: a language and environment for statistical computing. 2004. Vienna: R Foundation for Statistical Computing.
25. Ho SM. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003. 1:73.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download