Abstract
Port-site metastases in gynecological malignancies subsequent to laparoscopy have been reported with an incidence of 1.1-16%. These metastases tend to be disappearing after primary debulking surgery and subsequent primary chemotherapy. Local resection, chemotherapy and/or radiotherapy have been defined in the management of these metastases with enhanced clinical success. However, in extremely rare cases these metastases were also defined very early during neoadjuvant chemotherapy. Herein, we present two ovarian cancer cases which are clinically diagnosed with port site metastasis during neoadjuvant chemotherapy following diagnostic laparoscopy. Although neoadjuvant chemotherapy is sometimes needed in cases of fully advanced ovarian cancers, port-site metastasis may be encountered during neoadjuvant chemotherapy. The possible poor prognosis of these patients, especially those who have ascites, should make us careful in performing diagnostic laparoscopy with preventive measures for port-site metastasis and to start the chemotherapy immediately.
Diagnostic laparoscopy as a tool to identify candidates for primary cytoreductive surgery among patients with advanced stage ovarian cancer has been suggested, but remains controversial [1]. It has been reported that diagnostic laparoscopy contributed to selecting patients for primary surgery giving optimal cytoreduction in 61-96% of cases [2-4]. The use of laparoscopy in the management of cancer patients has brought many advantages such as shorter recovery time, improved performance, potential to initiate neoadjuvant therapy earlier, and decreased risk of developing adhesions. However, there are also disadvantages of tumor recurrences and metastases at the trocar insertion sites [5]. In 1978, port-site metastasis was defined subsequent to laparoscopy for the first time in an ovarian cancer patient by Dobronte et al. [6] The incidence of port-site metastasis for gynecological cancers is defined between 1.1-16% per procedure [7-10]. Herein, we present two cases with port-site metastasis diagnosed during neoadjuvant chemotherapy prior to primary surgery regardless of chemotherapy.
A 63 year-old menopausal woman referred with complaints of abdominal distention, vaginal bleeding, and loss of appetite. Ultrasonography revealed bilaterally enlarged ovaries (right 13×8×8 cm, left 12×10×9 cm) with multiple cystic and solid lesions and intra-abdominal ascites. The CA-125 level was 2,340 U/mL and the other tumor markers were within the normal range. Subsequently, a diagnostic laparoscopy was performed to evaluate operability of the patient as she had large bulky masses on ultrasonography. The laparoscopy revealed massive ascites, enlarged ovaries, and multiple diaphragmatic and peritoneal implants, along with an omental cake, and multiple biopsies were taken from the ovaries, omental cake, and peritoneal implants. There were 3 ports at the umbilicus (10 mm) and bilateral inguinal areas (both 5 mm), and the harvesting port was the left inguinal one. The pathological assessment revealed serous papillary ovarian cancer and multiple metastases to the peritoneal and serosal surfaces. Subsequently, neoadjuvant chemotherapy comprising cisplatinum (75 mg/m2) and paclitaxel (175 mg/m2) for 4 courses was given. CA-125 levels were measured as 1,999 U/mL, 944 U/mL, 368 U/mL and 160 U/mL at the chemotherapy courses, respectively. A subcutaneous, 2×1 cm diameter nodule was noticed on the 3rd neoadjuvant chemotherapy course, 47 days after the laparoscopic procedure, regardless of treatment. Subsequent to the 4th neoadjuvant chemotherapy course, primary debulking surgery including excision of port-site metastasis was performed, and the residual tumor size was <1 cm. The pathological assessment revealed poorly differented serous papillary ovarian cancer with invasion of bilateral ovarian surfaces, parametrium, serosal surfaces of tubes, uterus and appendix, as well with multiple invasions of the omentum. Furthermore, metastases of the periaortic (1/4) and right pelvic (1/6) lymph nodes were detected. Metastasis to the port-site was also diagnosed with pathological assessment of the 21×14 mm skin and subcutaneous tissue specimen which was excised from the right inguinal area 5 mm port-site. The patient had 6 courses of adjuvant chemotherapy consisting of cisplatinum (75 mg/m2) and paclitaxel (175 mg/m2) subsequent to the surgery. After the last course CA-125 levels were 32 U/mL. Thereafter patient was taken to the follow-up program and she had no signs of recurrence 12 months after the last adjuvant chemotherapy course.
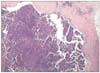
A 66 year-old woman was referred with a complaint of abdominal distention. Her initial gynecological and transvaginal ultrasonographic examination revealed bilateral enlarged ovaries (right 7×6×5 cm, left 6×6×9 cm) with multiple cystic and solid lesions and intra-abdominal ascites. The CA-125 level was 954 U/mL and the other tumor markers were normal. The cytological analysis of the ascites revealed an adenocarcinoma. Therefore, a diagnostic laparoscopy was performed to evaluate operability of the patient as she had a frozen pelvis on the bimanual gynecological examination and massive ascites, bilaterally enlarged ovaries, multiple implants of the diaphragm, peritoneal and serosal surfaces were determined, and multiple biopsies were taken from the ovaries and peritoneal surfaces. The three ports used during the procedure were 10 mm at the umbilicus and 5 mm at the bilateral inguinal areas. The harvesting port was the left inguinal one. The pathological assessment revealed a serous papillary ovarian cancer and multiple metastases to the peritoneal and serosal surfaces. Subsequently, neoadjuvant chemotherapy comprising of cisplatinum (75 mg/m2) and paclitaxel (175 mg/m2) was given for 4 courses with 21 day intervals. The level of CA-125 was 160 U/mL after the last chemotherapy course. A paraumbilical, 3×2 cm diameter subcutaneous nodule was noticed at the 10 mm port site after the second neoadjuvant chemotherapy course, 28 days after the laparoscopic procedure. Neoadjuvant chemotherapy was continued and the patient underwent primary debulking surgery. Port-site metastasis was also excised and the residual tumor size was <1 cm. The pathological assessment revealed serous papillary adenocarcinoma of the ovary with invasion of bilateral ovarian surfaces, parametrium, serosal surfaces of tubes, uterus and appendix as well multiple invasions of the omentum. Furthermore, metastases to pelvic (2/4) lymph nodes were reported. Metastasis to the port-site was also diagnosed by pathological assessment (Fig. 1). The patient had 6 courses of adjuvant chemotherapy consisting of cisplatinum (75 mg/m2) and paclitaxel (175 mg/m2) subsequent to the surgery. After the last course CA-125 levels were 35 U/mL. Thereafter the patient was taken to the follow-up program and she had no signs of recurrence 6 months after the last adjuvant chemotherapy course.
Diagnostic laparoscopy has become a preferred technique to evaluate operability of ovarian cancers. But one of the major concerns of laparoscopy in ovarian cancers is the development of port-site metastasis. Port site metastasis after diagnostic laparoscopy during neoadjuvant chemotherapy is an extremely rare situation [11]. In the vast majority (71%) of the cases the metastasis was isolated to and diagnosed at the "manipulation port" which is described as the port whereby instruments for biopsies inserted or extraction of tumor biopsies were done [12]. The median time to development of port-site metastasis was calculated as 17 days (range, 4 to 730 days) [12].
The potential risk factors for port-site metastasis are insufflation with carbon dioxide (tissue acidosis), high efflux of gas from the abdominal cavity through the space around the trocars (chimney effect), influence of local immune system, surgical technique, and the potential for contamination of the trocar site with viable tumor cells and presence of ascites [12,13]. According to Wang et al. [14] presence of ascites is significantly associated with the early occurrence of port-site metastases. There are some recommended measures to prevent port-site metastasis which include using wound protectors, minimal tumor manipulation, avoiding CO2 leaks and sudden desufflations, performing gasless laparoscopy, irrigation of ports with heparin or povidone-iodine solution before removal, excision of trocar sites and deliberate closure of all abdominal layers including the peritoneum, and early chemotherapy [9]. However, there is not enough evidence of effective prevention of port-site metastasis [9]. In Table 1 the two patients are summarized with risk factors for port-site metastasis.
Although there are reports about poor outcome with port-site metastasis during neoadjuvant chemotherapy in patients who have ascites, it has also reported that port-site metastasis do not affect the prognosis [8,12,13]. According to the study of van Dam et al. [8], port-site metastasis during neoadjuvant chemotherapy does not have a significant impact on the outcome. However, according to the study of Huang et al. [13], those patients who had port-site-metastasis that develop during chemotherapy have poor prognosis, and all died because of the cancer. The management of port-site metastases is primary excision of the tumor during debulking surgery. Despite the presence of ascites and occurrence during neoadjuvant chemotherapy, the treatment seems to be successful in our patients for the short duration of follow-up (Table 1).
The role of laparoscopy in ovarian malignancies is still a matter of debate. Although it has many benefits, there is a risk of port-site metastasis especially in advanced ovarian cancer patients who have ascites. Therefore, we should try to perform the preventive measures in practice as much as possible, and because of its possible poor prognosis we should start neoadjuvant chemotherapy course immediately. Anyhow, if port-site metastasis occurs the management should be local excision during debulking surgery.
Figures and Tables
References
1. Brun JL, Rouzier R, Uzan S, Darai E. External validation of a laparoscopic-based score to evaluate resectability of advanced ovarian cancers: clues for a simplified score. Gynecol Oncol. 2008. 110:354–359.
2. Vergote I, De Wever I, Tjalma W, Van Gramberen M, Decloedt J, van Dam P. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol. 1998. 71:431–436.
3. Angioli R, Palaia I, Zullo MA, Muzii L, Manci N, Calcagno M, et al. Diagnostic open laparoscopy in the management of advanced ovarian cancer. Gynecol Oncol. 2006. 100:455–461.
4. Fagotti A, Fanfani F, Ludovisi M, Lo Voi R, Bifulco G, Testa AC, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecol Oncol. 2005. 96:729–735.
5. Carlson NL, Krivak TC, Winter WE 3rd, Macri CI. Port site metastasis of ovarian carcinoma remote from laparoscopic surgery for benign disease. Gynecol Oncol. 2002. 85:529–531.
6. Dobronte Z, Wittmann T, Karacsony G. Rapid development of malignant metastases in the abdominal wall after laparoscopy. Endoscopy. 1978. 10:127–130.
7. Childers JM, Aqua KA, Surwit EA, Hallum AV, Hatch KD. Abdominal-wall tumor implantation after laparoscopy for malignant conditions. Obstet Gynecol. 1994. 84:765–769.
8. van Dam PA, DeCloedt J, Tjalma WA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999. 181:536–541.
9. Nagarsheth NP, Rahaman J, Cohen CJ, Gretz H, Nezhat F. The incidence of port-site metastases in gynecologic cancers. JSLS. 2004. 8:133–139.
10. Kruitwagen RF, Swinkels BM, Keyser KG, Doesburg WH, Schijf CP. Incidence and effect on survival of abdominal wall metastases at trocar or puncture sites following laparoscopy or paracentesis in women with ovarian cancer. Gynecol Oncol. 1996. 60:233–237.
11. Vergote I, Marquette S, Amant F, Berteloot P, Neven P. Port-site metastases after open laparoscopy: a study in 173 patients with advanced ovarian carcinoma. Int J Gynecol Cancer. 2005. 15:776–779.
12. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003. 91:179–189.
13. Huang KG, Wang CJ, Chang TC, Liou JD, Hsueh S, Lai CH, et al. Management of port-site metastasis after laparoscopic surgery for ovarian cancer. Am J Obstet Gynecol. 2003. 189:16–21.
14. Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999. 72:38–44.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download