Abstract
Purpose
Among cell adhesion molecules, serum levels of intercellular adhesion molecule-1 and E-selectin are known to be correlated with the metastatic potential of gastric cancer. In the present study, the authors investigated the expression of intercellular adhesion molecule-1 and E-selectin in gastric cancer tissues and cultured gastric cancer cells, and examined their clinical value in gastric cancer.
Materials and Methods
The protein was extracted from gastric cancer tissues and cultured gastric cancer cells (MKN-28 and Kato-III) and the expression of intercellular adhesion molecule-1 and E-selectin was examined by western blotting. The clinical significance of intercellular adhesion molecule-1 and E-selectin was explored, using immunohistochemical staining of specimens from 157 gastric cancer patients.
Results
In western blot analysis, the expressions of intercellular adhesion molecule-1 in gastric cancer tissues and cultured gastric cancer cells were increased, however, E-selectin in gastric cancer tissues and cells were not increased. Among 157 gastric cancer patients, 79 patients (50%) were intercellular adhesion molecule-1 positive and had larger tumor size, an increased depth of tumor invasion, lymph node metastasis and perineural invasion. The intercellular adhesion molecule-1 positive group showed a higher incidence of tumor recurrence (40.5%), and a poorer 3-year survival than the negative group (54.9 vs. 85.9%, respectively).
Conclusions
Intercellular adhesion molecule-1 is overexpressed in gastric cancer tissues and cultured gastric cancer cells, whereas E-selectin is not overexpressed. Increased expression of intercellular adhesion molecule-1 in gastric cancer could be related to the aggressive nature of the tumor, and has a poor prognostic effect on gastric cancer.
The adhesion between endothelial cells and cancer cells is essential for cancer invasion and this process is mediated by intercellular adhesion molecules including intercellular adhesion molecule-1 (ICAM-1) and E-selectin.(1,2) There are three distinct steps in the process of cancer cell metastasis, including detachment of the cancer cells from the primary lesion, penetration through the tissue basement membrane, invasion of blood vessels and migration to the target organ.(3) Concerning the detachment of cancer cells, an increase in metastatic potential has been reported along with the reduction of cell adhesion molecules.(4) Therefore, some researchers have reported a negative relationship between ICAM-1 expression in cancer cells and tumor progression or metastasis.(5,6)
Generally, host defense mechanisms of immune cells are considered to play an important role in suppression of cancer metastasis. ICAM-1, which suppresses the function of immune cells by binding to lymphocytes, may act as a kind of immunosuppressive substance.(7-9) Therefore, with respect to the relationship between cancer cells and ICAM-1, other researchers have reported that elevation of ICAM-1 expression in cancer cells and increased release of serum ICAM-1 from cancer cells are related with cancer progression or metastasis.(10-12)
Selectins are adhesion molecules that mediate the initial binding of leukocytes to microvascular endothelium by lectin-type interactions with carbohydrate ligands on corresponding target cells.(13,14) E-selectin is detected on the surface of endothelial cells upon activation by cytokines and binds to target cell surfaces by oligosaccharide recognition.(15) The binding of cancer cells to endothelial cells by E-selectin is reportedly related to their metastatic potential.(16)
In gastric cancer, some researchers have reported that a negative relationship between ICAM-1 expression in gastric cancer cells and tissues and cancer progression or metastatic potential which has a better prognostic effect.(17,18) Otherwise, others have reported a positive relationship between serum levels of circulating ICAM-1, E-selectin and their expression in gastric cancer cells and tumor progression and metastatic potential, which has a poor prognostic effect.(19-22)
In the present study, the authors investigated the expression of ICAM-1 and E-selectin in gastric cancer tissues and cultured gastric cancer cells, and examined their prognostic value in gastric cancer patients.
Gastric cancer cell lines (MKN28, KATOIII) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 µg/ml streptomycin and 100 unit/ml penicillin and incubated at 37℃ in a humidified atmosphere containing 5% CO2 in air (referred to as the normoxic condition).
To obtain the intestinal proteins, cells and tissues were homogenized in RIPA buffer (20 mM Tris, 1 mM EDTA, 255 mM sucrose, pH 7.1). The supernatants were used. The protein concentrations were determined using a PIERCE Protein Assay Regent (Pierce, Rockford, IL, USA). Each protein sample was mixed with Laemmli sample buffer (Bio-Rad, Hercules, CA, USA). Western blots were performed using 20 µg of total protein. Protein samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 10% acrylamide gels. The proteins were transferred onto nitrocellulose membranes (Protran, Whatman, Dassel, Germany), which were blocked in 5% non-fat dry milk in TBS buffer for 1 hour. Then, the nitrocellulose membrane was incubated with a polyclonal antibody against E-selectin (Biovision, Inc., Milpitas, CA, USA, 1 : 1,000) and ICAM-1 (SANTACRUZ, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, 1 : 100) diluted in blocking solution overnight at 4℃. Afterwards, the membranes were rinsed three times with TBS, 0.1% Tween-20 for 10 minutes, and re-incubated for 1 hour at room temperature in blocking buffer with 1 : 2,500 diluted horseradish peroxidase-conjugated secondary antibody, and then washed three times for 10 minutes. Protein detection was performed using the electrochemiluminescence Plus system (Amersham Biosciences, GE Healthcare, Pittsburgh, CT, USA). Optical density analysis of proteins was performed using Image J software (download directory at "imagej.nih.gov/ij/download/."). Image J is a public domain Java image processing program produced by National Institute of Health. The negative control was the zero signal density, which was the amount of E-selectin protein in KATOIII cells. The reference level was an amount of protein expression in normal stomach tissue determined as one. The amount of protein expression levels in other gastric cancer tissue and cells were measured by the proportion with the amount of density in normal stomach tissue level.
We studied 157 patients with gastric cancer who underwent gastrectomy at Korea University Hospital from 2003 to 2006. The mean follow-up period was 35.8 months (range, 15~69 months). The clinical data, including gender, age, and cancer recurrence including distant metastasis were obtained from medical records and the data on tumor size, depth of tumor invasion, lymph node metastasis, Lauren classification, lympho-vascular invasion and perineural invasion were obtained from pathology reports. Before designing this study, we received the approval of the Institutional Review Board in Korea University Hospital during the process of specimen and data collection (Approval number: AS0825-001).
Tissue micro-array was made for the primary tumors of all patients. H&E stained slides of primary tumor tissue were reviewed and central portion and invasion front of tumor was selected. Tumor tissue at the marked portion was aspirated from the paraffin block by Quik-Ray™ SYSTEM (Unitma Co., Ltd., Seoul, Korea, Core needle biopsy). Two cores were cut from each donor block for the tissue microarray. Five tissue array blocks were prepared and each contained about 60 tumor cores. Sections of 5 mm thickness were cut and processed for ICAM-1 and E-selectin immunostaining. Sections were stained for ICAM-1 and E-selectin antigen using the avidin-biotin-peroxidase complex method. We used ICAM-1 rabbit monoclonal antibody (Epitomics, Inc., Burlingame, CA, USA) and E-selectin polyclonal antibody (Biovision, Inc.) as primary antibodies. After deparaffinization in xylene and washing in ethanol, sections were heated in 0.01 M sodium citrate buffer (pH 6) at 95℃ for 20 minutes to unmask hidden epitopes.
After cooling to room temperature and wash in 0.01 M phosphate-buffered saline, the slides were processed in DAKO EnVsion™+/HRP kit according to the manufacturer's instructions (Copenhagen, Denmark). The ICAM-1 monoclonal antibody was diluted 1 : 100 in phosphate-buffered saline and E-selectin polyclonal antibody was diluted 1 : 1,000, and then they were reacted with tissue specimens at 37℃ for 30 minutes. Diaminobenzidine was used as a chromogen, followed by hematoxylin counterstaining. Normal rabbit IgG was substituted for primary antibody as the negative control. For positive controls, staining patterns of endothelial cells or leukocytes were checked. The specimens were examined under the microscope. When the tumor specimens were reacted with anti-ICAM-1 and E-selectin antibody, they showed various staining patterns. The degree of positive staining of tumor cells was represented as an approximate percentage of positive cells. As Yashiro et al.(18) indicated, for analyses, specimens were considered positive if more than 50% of the cancer cells expressed ICAM-1 and E-selectin. Specimens with less than 50% staining of cancer cells were classified into the ICAM-1 and E-selectin-negative groups.
The statistical significance of the correlation between ICAM-1 expression and clinicopathologic features was evaluated by chi-square test and independent t-test. Survival curves were constructed according to the Kaplan-Meier method. For statistical differences between curves, a P-value was calculated using the log-rank test. A Cox regression model was used to examine independent prognostic factors in patients. All statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA) and a P-value<0.05 was considered statistically significant.
Western blotting was performed using gastric cancer tissues as well as corresponding tissues from non-cancerous regions and two gastric cancer cell lines, MKN28 and KATO-III. ICAM-1 expression was noted in normal stomach tissues and the level of expression was increased as much as four times in gastric cancer tissues compared with normal stomach tissues. However, E-selectin expression was more prominent in normal stomach tissues and it was decreased in gastric cancer tissues. In cultured gastric cancer cell lines, MKN28 and KATO-III, the level of expression of ICAM-1 was increased, especially in MKN28, but the level of expression of E-selectin was not increased (Fig. 1).
From 157 gastric cancer tissues, nearly 50% (n=79) tissues were positive for ICAM-1 and 54% (n=85) were positive for E-selectin by immunohistochemical staining (Fig. 2). There was a reverse relationship between the positivity of ICAM-1 expression and positivity of E-selectin expression (Table 1).
In our analysis, there was no statistically significant relationship between the expression of E-selectin and clinicopathologic factors and prognosis in these gastric cancer patients.
Statistical analysis showed that the ICAM-1 positive group had a larger tumor size, advanced depth of tumor invasion, positive lymph node involvement and distant metastasis, namely advanced cancer stage. And hence, the expression of ICAM-1 was significantly related with the positivity of perineural invasion (Table 2).
In the relationship between ICAM-1 and cancer recurrence, ICAM-1 was significantly correlated with cancer recurrence and when divided according to the recurrence pattern, it was mostly associated with peritoneal seeding (56.3%, 18/32 cases) (Table 3).
In survival analysis, ICAM-1-positive group showed a poor 3-year survival than the ICAM-1-negative group. (54.9% vs. 85.9%) and the disease-free survival result was also the same (Fig. 3).
In analysis of prognostic factors in these patients, ICAM-1 positive expression, diffuse type, depth of tumor invasion, lymph node involvement, distant metastasis, lymphovascular invasion and perineural invasion were proved to be negative prognostic factors in univariate analysis. However, in multivariate analysis, depth of tumor invasion and distant metastasis were proved to be independent prognostic factors (Table 4).
In western blot assay, we could identify the presence of two adhesion molecules (ICAM-1 and E-selectin) in normal stomach tissues and gastric cancer tissues. Interestingly, the expression of ICAM-1 was increased in gastric cancer tissues compared with normal stomach tissues, but the E-selectin expression was decreased in gastric cancer tissues. Furthermore, in the cultured gastric cancer cells, ICAM-1 protein expression was noted but E-selectin protein expression was not noted.
These results can be explained by the hypothesis that E-selectins are located on the surface of gastric endothelial cells as adhesion molecules in normal gastric tissue, and they maybe lost their binding effect on the cell to cell interaction during the carcinogenesis. Therefore they could not show any expression in gastric cancer cells and decreased in gastric cancer tissue. Otherwise, we can suppose that ICAM-1 plays a more crucial role in carcinogenesis and tumor progression in gastric cancer compared to E-selectin.
ICAM-1 (CD54), a member of immunoglobulin superfamily, is a transmembrane glycoprotein with an extracellular region that has an immunoglobulin-like structure.(23) ICAM-1 is found on the surface of numerous cells including vascular endothelial cells, fibroblasts, and epithelial cells.(24) After binding to lymphocyte function antigen-1 (LFA-1) on the surface of T lymphocytes, it plays a role in their activation that increases the susceptibility of such tumor cells to lymphocyte-mediated tumor cytotoxicity.(25) ICAM-1 on cancer cells plays an important role in adhering to natural killer (NK) cells or lymphocyte-activated killer (LAK) cells via LFA-1.(26) Migration of granulocytes from blood vessels is mediated by adhesion between ICAM-1 and LFA-1(27) and ICAM-1 is also involved in adhesion to target cells before destruction by killer cells (NK cells, LAK cells).(28) Therefore, ICAM-1 is important for the local immune response, i.e. the activity of NK and LAK cells is suppressed by administration of anti-ICAM-1 antibody(8,9) and serum circulating ICAM-1 suppresses the adhesion of T cells by binding to LFA-1 and inhibits NK and LAK cell activity.(7,29) Based on these reports, we can hypothesize that ICAM-1 has an important immunosuppressive effect and is involved in carcinogenesis and aggravates cancer progression and this can be applied to our analysis.
In the past, there have been several reports that demonstrated the protective effect of ICAM-1 protein in gastric carcinogenesis and cancer progression.(17,18) Fujihara et al.(17) documented lymph node metastasis as a significant risk factor for gastric cancer and proposed that ICAM-1 promotes immunosurveillance system between cancer cells and cytotoxic lymphocytes which are associated with blocking the lymph node metastatic process. Also, in the mechanism of immunologic tolerance, the escape of cancer cells from immune system can be responsible for progression of cancer. Yashiro et al.(18) suggested that ICAM-1 could be a costimulatory molecule which plays an important role in cancer cell adhesion to immune cells for this immune tolerance, and hence decreased expression of ICAM-1 on cancer cells might be associated with decreased cytotoxicity of immune cells, which may result in lymph node metastasis and tumor progression in gastric cancer.
However, most other reports regarding the importance of serum circulating ICAM-1 in gastric cancer suggested that increased serum levels of ICAM-1 in gastric cancer may reflect cancer progression and metastasis.(19,20,22) Benekli et al.(20) demonstrated that concentrations of serum circulating ICAM-1 are increased in gastric cancer patients with distant and peritoneal metastasis and they suggested that ICAM-1 could be a reflective metastatic marker for gastric cancer. Also, Maruo et al.(22) proposed that ICAM-1 was over-expressed in cancer cells and tissues and it could be released as a serum circulating ICAM-1, which would promote hematogenous metastasis by suppressing local antitumor immunity in gastric cancer. Considering the above two conflicting opinions, the role of ICAM-1 as a prognostic marker in gastric cancer has not yet been established because there is a reciprocal effect of ICAM-1 on immune surveillance system which is like two sides of the same coin. These two conflicting results of previous reports are summarized in Table 5.
Although we could not obtain the serum levels of circulating ICAM-1 because we used a retrospective study design, we could identify the presence of ICAM-1 in gastric cancer tissues comprised of different types of cells including endothelial cells, lymphocytes, monocytes and other immune cells and they could be a source of serum circulating ICAM-1. And in our analysis, patients with increased expression of ICAM-1 showed an advanced tumor stage and a more frequent incidence of cancer recurrence and peritoneal seeding was the most common form of metastasis. Therefore, these results reconfirm that ICAM-1 in cancer tissues may have an immunosuppressive effect due to the escape of cancer cells from immune surveillance system which is the similar action of serum circulating ICAM-1 in previous other studies.
Although the negative prognostic effect of ICAM-1 on hematogenous metastasis in gastric cancer has been emphasized in the previous report,(22) considering that peritoneal seeding is the most common recurrence pattern in gastric cancer, our result can be explained by the general effect of ICAM-1 protein on tumor progression and metastasis in gastric cancer patients.
In our immunohistochemical staining analysis, we could not demonstrate any meaningful relationship between the expression of E-selectin and tumor progression. However, interestingly, a reverse correlation between ICAM-1 and E-selectin protein expression was noted and this was similar to the finding of western blot analysis. A further study regarding the carcinogenetic and prognostic role of E-selectin protein in gastric cancer is needed. Several basic researches about the function and effect of ICAM-1 in immunology and carcinogenesis were described,(23-28) however, in gastric cancer, the basic research for investigating the role of ICAM-1 on gastric carcinogenesis and prognosis could not yet be performed. So, we think this basic fundamental study should be needed. Also, more detailed invasion and migration study or proliferation study of gastric cancer cells with or without ICAM-1 inhibitor should be needed for clarification of mechanism in gastric carcinogenesis and cancer progression.
In our gastric cancer patients, we could not demonstrate ICAM-1 as an independent prognostic factor in multivariate analysis due to the powerful impact of cancer stage on survival. Although subgroup analysis was not possible because the sample size of our study was small, we think that there is a significant possibility of ICAM-1 as the independent prognostic factor in gastric cancer, especially in the advanced stage, when a large-scale, multicenter research study will be undertaken.
As significant progress has been made recently in molecular tumor biology, new therapeutic approaches such as immune-based and targeted therapies in gastric cancer have been intensively investigated.(30) Considering the importance of immune system affecting tumor progression and metastasis, immune therapeutic effects of ICAM-1 and/or anti-ICAM-1 antibody in gastric cancer are worthy of careful study in the future.
In conclusion, the present study demonstrated that ICAM-1 is over-expressed in the gastric cancer tissues and cultured gastric cancer cells compared with normal stomach tissues, and gastric cancer patients with high expression of ICAM-1 showed an advanced tumor stage and metastasis. Therefore, the increased expression of ICAM-1 protein in tumor tissues from pathology specimens could be an additional prognostic indicator to monitor for cancer recurrence along with increased serum levels of circulating ICAM-1 during the postoperative follow-up in gastric cancer patients.
Figures and Tables
Fig. 1
Intercellular adhesion molecule-1 (ICAM-1), E-selectin expression level in gastric cancer tissues and cell lines (MKN28, KATO3). (A) CAM-1 expression was noted in normal stomach tissues but it was increased in gastric cancer tissues and cultured gastric cancer cells. (B) E-selectin expression was more prominent in normal stomach tissues but it was decreased in gastric cancer tissues and cultured gastric cancer cells.
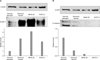
Fig. 2
Immunohistochemical staining of intercellular adhesion molecule-1 (ICAM-1), E-selectin in tissue microarray slides of gastric cancer. (A) Expression of ICAM-1 was noted in the endothelial cells of gastric cancer tissues. More than 50% of cancer cells were stained (×400). (B) Expression of E-selectin was noted in the diffuse type signet ring gastric cancer cells. More than 50% were stained (×400).

Fig. 3
(A) Overall 3-year survival of gastric cancer patients according to the intercellular adhesion molecule-1 (ICAM-1) immune reactivity. (B) Disease-free 3-year survival of gastric cancer patients according to the ICAM-1 immune reactivity.

Table 2
The relationship between ICAM-1 expression and clinicopathologic factors in gastric cancer patients

Acknowledgments
This work was supported by Korea University Grant.
This manuscript was presented by poster on March, 2012 at the 33rd Annual Spring Congress of Korean Gastric Cancer Association in Kimdaejung Convention Center, Kwangju, Korea.
References
1. Ohene-Abuakwa Y, Pignatelli M. Adhesion molecules as diagnostic tools in tumor pathology. Int J Surg Pathol. 2000. 8:191–200.

3. Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992. 10:561–591.

4. Sommers CL, Thompson EW, Torri JA, Kemler R, Gelmann EP, Byers SW. Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities. Cell Growth Differ. 1991. 2:365–372.
5. Maeda K, Kang SM, Sawada T, Nishiguchi Y, Yashiro M, Ogawa Y, et al. Expression of intercellular adhesion molecule-1 and prognosis in colorectal cancer. Oncol Rep. 2002. 9:511–514.

6. Ogawa Y, Hirakawa K, Nakata B, Fujihara T, Sawada T, Kato Y, et al. Expression of intercellular adhesion molecule-1 in invasive breast cancer reflects low growth potential, negative lymph node involvement, and good prognosis. Clin Cancer Res. 1998. 4:31–36.
7. Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. A form of circulating ICAM-1 in human serum. J Immunol. 1991. 147:3788–3793.
8. Wang P, Vánky F, Li SL, Patarroyo M, Klein E. Functional characteristics of the intercellular adhesion molecule-1 (CD54) expressed on cytotoxic human blood lymphocytes. Cell Immunol. 1990. 131:366–380.

9. Sánchez-Rovira P, Jimenez E, Carracedo J, Barneto IC, Ramirez R, Aranda E. Serum levels of intercellular adhesion molecule 1 (ICAM-1) in patients with colorectal cancer: inhibitory effect on cytotoxicity. Eur J Cancer. 1998. 34:394–398.

10. Tsujisaki M, Imai K, Hirata H, Hanzawa Y, Masuya J, Nakano T, et al. Detection of circulating intercellular adhesion molecule-1 antigen in malignant diseases. Clin Exp Immunol. 1991. 85:3–8.

11. Natali P, Nicotra MR, Cavaliere R, Bigotti A, Romano G, Temponi M, et al. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990. 50:1271–1278.
12. Koyama S, Ebihara T, Fukao K. Expression of intercellular adhesion molecule 1 (ICAM-1) during the development of invasion and/or metastasis of gastric carcinoma. J Cancer Res Clin Oncol. 1992. 118:609–614.

13. Bevilacqua M, Butcher E, Furie B, Furie B, Gallatin M, Gimbrone M, et al. Selectins: a family of adhesion receptors. Cell. 1991. 67:233.

15. Majuri ML, Mattila P, Renkonen R. Recombinant E-selectin-protein mediates tumor cell adhesion via sialyl-Le(a) and sialyl-Le(x). Biochem Biophys Res Commun. 1992. 182:1376–1382.

16. Ramphal JY, Hiroshige M, Lou B, Gaudino JJ, Hayashi M, Chen SM, et al. Ligand interactions with E-selectin. Identification of a new binding site for recognition of N-acyl aromatic glucosamine substituents of sialyl Lewis X. J Med Chem. 1996. 39:1357–1360.

17. Fujihara T, Yashiro M, Inoue T, Sawada T, Kato Y, Ohira M, et al. Decrease in ICAM-1 expression on gastric cancer cells is correlated with lymph node metastasis. Gastric Cancer. 1999. 2:221–225.

18. Yashiro M, Sunami T, Hirakawa K. CD54 expression is predictive for lymphatic spread in human gastric carcinoma. Dig Dis Sci. 2005. 50:2224–2230.

19. Yoo NC, Chung HC, Chung HC, Park JO, Rha SY, Kim JH, et al. Synchronous elevation of soluble intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) correlates with gastric cancer progression. Yonsei Med J. 1998. 39:27–36.

20. Benekli M, Güllü IH, Tekuzman G, Savaş MC, Hayran M, Hasçelik G, et al. Circulating intercellular adhesion molecule-1 and E-selectin levels in gastric cancer. Br J Cancer. 1998. 78:267–271.

21. Ke JJ, Shao QS, Ling ZQ. Expression of E-selectin, integrin beta1 and immunoglobulin superfamily member in human gastric carcinoma cells and its clinicopathologic significance. World J Gastroenterol. 2006. 12:3609–3611.

22. Maruo Y, Gochi A, Kaihara A, Shimamura H, Yamada T, Tanaka N, et al. ICAM-1 expression and the soluble ICAM-1 level for evaluating the metastatic potential of gastric cancer. Int J Cancer. 2002. 100:486–490.

23. Staunton DE, Marlin SD, Stratowa C, Dustin ML, Springer TA. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988. 52:925–933.

24. Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986. 137:245–254.
25. Staunton DE, Dustin ML, Erickson HP, Springer TA. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990. 61:243–254.

26. Schmidt RE, Bartley G, Levine H, Schlossman SF, Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985. 135:1020–1025.
27. Dustin ML, Springer TA. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991. 9:27–66.

28. Vánky F, Wang P, Patarroyo M, Klein E. Expression of the adhesion molecule ICAM-1 and major histocompatibility complex class I antigens on human tumor cells is required for their interaction with autologous lymphocytes in vitro. Cancer Immunol Immunother. 1990. 31:19–27.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download