Abstract
Purulent pericarditis is a rare condition with a high mortality rate. We report a case of purulent pericarditis subsequently caused by Candida parapsilosis, Peptostreptococcus asaccharolyticus, Streptococcus anginosus, Staphylococcus aureus, Prevotella oralis, and Mycobacterium tuberculosis in a previously healthy 17-year-old boy with mediastinal tuberculous lymphadenitis. The probable route of infection was a bronchomediastinal lymph node-pericardial fistula. The patient improved with antibiotic, antifungal, and antituberculous medication in addition to pericardiectomy.
Purulent pericarditis is a rare condition [1] that occurs most commonly from adjacent infectious intrathoracic lesions such as those due to pneumonia, empyema, myocarditis, and endocarditis [2]. Hematogenous spread from distant infections, a perforating injury, or direct inoculation during thoracic surgery or following catheter drainage are also possible pathogenic mechanisms, which lead to secondary purulent pericarditis [34]. The most common pathogen is Staphylococcus aureus, but cases of polymicrobial purulent pericarditis are occasionally reported as well [45678]. Polymicrobial purulent pericarditis develops most often after procedures [67], but primary infections in patients with esophageal cancer, diabetes, and human immunodeficiency virus (HIV) infection have also been reported [58]. A case of purulent pericarditis due to infection with Streptococcus pneumoniae and Mycobacterium tuberculosis in an HIV patient was reported in South Africa [9], but there have been no previous reports of polymicrobial pericarditis occurring as a complication of tuberculosis (TB) in an immunocompetent patient. To our knowledge, this is the first report of bacterial and fungal pericarditis to have occurred during mediastinal tuberculous lymphadenitis and pericarditis. In this report, we present a case of purulent pericarditis subsequently caused by Candida parapsilosis, Peptostreptococcus asaccharolyticus, Streptococcus anginosus, Staphylococcus aureus, Prevotella oralis, and Mycobacterium tuberculosis in a previously healthy 17-year-old boy with MTL.
A 17-year-old boy presented to the emergency room (ER) complaining of shortness of breath and pleuritic chest pain that had developed 2 hours earlier. The patient was a high school student who had been well until 1 month before this visit. In the subsequent month, he had a dry cough, fatigue, and weight loss of 2 kg. He had no febrile sensation or myalgia. The day before, he developed fever, chills, and pleuritic chest pain rated 3 points on a 0-10 numeric pain intensity scale. The pain was located on the anterior chest wall and was aggravated when the patient inspired deeply. Two hours before visiting the ER, shortness of breath developed, and the pleuritic chest pain was aggravated and radiated to the shoulder and jaw. He did not smoke nor drink alcohol, and did not have any trauma.
The patient was alert but appeared acutely ill. Vital signs included a body temperature of 39.0℃, blood pressure of 107/70 mmHg, heart rate of 110 beats/min, respiratory rate of 30/min, and 98% oxygen saturation with pulse oximetry on room air. Results of examinations of the head, eye, ear, nose, and throat were unremarkable. The jugular vein was not distended. On chest examination, lung auscultation was normal, but a rapid and distant heart sound was heard. On abdominal examination, there was no tender point or organomegaly. No pitting edema on the peripheral limbs was observed.
Initial laboratory findings were as follows: white blood cell count was 20,100/mm3 with 88.4% neutrophils, hemoglobin 12.6 g/dL, hematocrit 37.3% and the platelet count was 394,000/mm3. Additional findings included the following: blood urea nitrogen, 7.2 mg/dL (reference range, 7-20 mg/dL); serum creatinine, 0.78 mg/dL (reference range, 0.6-1.4 mg/dL); C-reactive protein, 21.7 mg/dL (reference range <0.3 mg/dL); aspartate aminotransferase, 84 U/L (reference range, 5-40 U/L); alanine aminotransferase, 177 U/L (reference range, 5-45 U/L), D-dimer, 2.97 mg/L (reference range, 0-0.24 mg/L); B-type natriuretic peptide, 97 pg/mL (reference range 0-100 pg/mL); and troponin I, 0.04 ng/mL (reference range 0.01-0.06 ng/mL). An HIV antibody test result was negative. On electrocardiography, sinus tachycardia (heart rate 116 beats/min), elevation of PR segment in aVR, and depression of PR in the other leads were observed. A chest radiograph showed cardiac silhouette enlargement with left lower lobar consolidation (Fig. 1). In a computed tomography (CT) scan of the chest, pericardial effusion with pericardial enhancement and multiple necrotic lymph nodes containing air in the mediastinal area were observed, which suggested a broncho-lymph nodal fistula (Fig. 2). Echocardiography showed a large amount of pericardial effusion with normal cardiac wall motion. TB pericarditis and lymphadenitis were presumed, and the patient was admitted to the intensive care unit. Intravenous ceftriaxone was administered from the first day of his arrival at the ER.
Six hours after admission, he began to breathe faster (40 breaths/min) and his blood pressure dropped to 90/50 mmHg. Jugular venous distention was observed. Cardiac tamponade was presumed, and emergency fluoroscopy-guided percutaneous pericardiocentesis was performed during which 200 mL of pus-like fluid was drained. The catheter was left in the pericardial space for drainage. Fluid specimens were sent for culture and chemical analysis. Chemical analysis findings of initial pericardial fluid were as follows: white blood cell count, 197,840/mm3 with 98% neutrophils; red blood cell count, 70,000/mm3, lactate dehydrogenase, 1,894 U/L; triglycerides, 31 mg/dL; glucose, 3 mg/dL; and adenosine deaminase, 91.8 U/L. Microbiologic results of the pericardial fluid culture are shown in Figure 3.
On the following day, a Mycobacterium tuberculosis polymerase chain reaction (TB PCR) test performed on the pericardial fluid yielded positive results. Acid-fast bacillus (AFB) staining and Gram staining were all negative. Isoniazid, rifampin, ethambutol, and pyrazinamide with pyridoxine were administered. On hospital day 3, we learned that yeast was growing in the initial pericardial fluid culture so we added intravenous fluconazole. On hospital day 4, Candida parapsilosis was identified in the initial pericardial fluid culture. The amount of fluid drained from the pericardial catheter decreased to 70 mL/day, but his fever persisted. We learned that gram-positive bacterium was growing in the pericardial fluid drained on the first hospital day. We used vancomycin and ciprofloxacin in addition to fluconazole as treatment for pyogenic pericarditis. On hospital day 7, the fever developed daily, and vital signs included a body temperature of 38.1℃, blood pressure of 110/75 mmHg, heart rate of 98 beats/min and respiratory rate of 18/min. Chest radiography showed that the cardiac silhouette had decreased in size but that pneumopericardium had developed (Fig. 4). Subsequent chest CT image showed that pneumopericardium newly developed and the pericardial wall became more thickened and septated (Fig. 5). We switched vancomycin and ciprofloxacin with vancomycin and ertapenem for the uncontrolled, mixed infection because of the pneumopericardium. On hospital day 9, Peptostreptococcus asaccharolyticus and Streptococcus anginosus in addition to C. parapsilosis were identified from the initial pericardial fluid culture. Methicillin-sensitive S. aureus and Prevotella oralis were also determined to the causative organisms of pyogenic pericarditis in this case, isolated respectively from pericardial fluid drained on hospital day 4 and hospital day 7. At this point, we understood that the multiple organisms in the pericardial fluid would be from the oral cavity through a broncho-lymph nodal fistula and possibly a lymph nodal-pericardial fistula. Upon reviewing the chest CT image, we observed that the subcarinal lymph node with air density was shown to contact with the parietal pericardium at coronal section images (panel D in Fig. 2). Because of persistent pericardial drainage, pericardial thickening, and probable broncho-lymph node-pericardial fistula, a pericardiectomy was performed on hospital day 10. Pus, inflammatory material, and multiple septations were observed within the pericardium during the procedure, and irrigation with suction and removal of septations were performed for smooth drainage of the pericardial fluid. There was neither gross pericardial fistula nor evidence of mediastinitis. In order to identify the suspected tracheobronchial fistula, an air leakage test was performed, but no leakage was observed. The thickened pericardium and epicardium were removed (mostly on the anterior right ventricle side). Therefore, subcarinal and paratracheal lymph nodes were not dissected. Pericardial tissue biopsy revealed acute and chronic inflammation with liquefactive necrosis. The patient began to improve postoperatively. TB cultures of the pericardial fluid were all negative. One month after admission, the patient was discharged with oral antifungal, antibiotic and anti-TB medication. We stopped amoxicillin/clavulanic acid after three months and fluconazole after six months, but patient remained on anti-TB medication. Six months after discharge, the patient returned to normal activity including playing soccer, and his heart rate was around 70 beats/min. Echocardiography showed no cardiac relaxation abnormality, although the ejection fraction was minimally decreased.
TB lymphadenitis is observed in nearly 35% of cases of extrapulmonary TB, which constitutes approximately 15-20% of all cases of TB [10]. The incidence of pericardial involvement among pulmonary TB is estimated to be 1-8% [11]. In most cases, TB pericarditis occurs due to the progression of infection into the pericardium from the mediastinal lymph nodes, particularly those at the tracheobronchial bifurcation [2]. In our case, chest CT showed large, necrotizing lymph nodes containing air at the site of the tracheobronchial bifurcation coming into contact with the pericardium.
This case was unusual in that bacteria, candida, and mycobacteria were all identified at the same time from the pericardial fluid of a healthy young boy, and that a pneumopericardium occurred by possible broncho-lymph node-pericardial fistula rather than by direct bronchopericardial fistula formation. At first, we believed that the culture result of Candida parapsilosis was likely due to contamination. When the bacteria serially isolated were all from the oral cavity and the pneumopericardium appeared, we understood the mechanism of this unusual result.
Initial Gram stain of the pericardial fluid was negative although the culture was positive, which may have been because of the administration of antibiotics before pericardiocentesis, or simply a false-negative result of the Gram stain. The false negative rate for Gram stain is reported to be 25-50% in septic arthritis [12], although the sensitivity of the Gram stain in pericardial fluid has not been reported.
When the pneumopericardium developed on hospital day 7, we considered three explanations for this; first, that it developed because of pericardial drainage; second, that gas formation occurred by uncontrolled mixed infection with anaerobic bacteria; and third, that the air leak was worsened through enlargement of the fistula. A cardiologist gave the opinion that a pneumopericardium was less likely to occur with pericardial drainage because the intrapericardial pressure was higher than the atmospheric pressure. We performed a pericardiectomy early because of possible persistent mixed infection and broncho-lymph node-pericardial fistula. However, because the surgeons could not find the gross fistula intraoperatively, lymph node dissection and fistula closure were not performed, and the patient recovered without further problems.
Cases of lymphobronchial fistulae in patients with TB lymphadenitis have been reported in children and HIV patients [1314]. Although rare, bronchopericardial fistulae can occur as a complication of tuberculosis, histoplasmosis, and aspergillosis [1516] as well as be caused by neoplasm [17], iatrogenesis [18], and trauma [19]. The treatment for a bronchopericardial fistula is stent insertion and surgery [17], although there is at least one case in the literature reporting spontaneous healing of a bronchopericardial fistula after pericardial drainage [14]. In our case, we speculate that the fistula must have been very small (a microperforation), but large enough for bacteria to pass through, and that it may have been closed when inflammation was decreased with antibiotics, antifungals and anti-TB medication in addition to appropriate drainage of inflammatory materials. The sequence in which the organisms were isolated may have been a function of organism invasiveness or of their abundance in the oral cavity.
In summary, we report a case of polymicrobial purulent pericarditis that occurred in a patient with mediastinal tuberculous lymphadenitis, with the probable mechanism being a bronchomediastinal lymph node-pericardial fistula.
Figures and Tables
Figure 1
Anteroposterior chest radiograph; the cardiac silhouette is globularly enlarged and looks like a "water bottle".
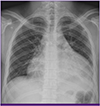
Figure 2
Chest computed tomography. Transverse section image shows a large amount of pericardial effusion with pericardial enhancement (panel A). Multiple necrotic lymph nodes containing air density are observed in the pretracheal (panel B, arrow) and subcarinal areas (panel C, arrow). Coronal section image shows subcarinal lymph node with air density in contact with the parietal pericardium (panel D, arrow).
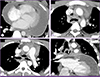
Figure 3
Hospital course of the patient and identified pathogens from pericardial fluid cultures. Hospital day 2: The TB PCR text was positive for the initial pericardial fluid and also positive for those samples obtained at day 4 and 10. Hospital day 2 to 4: Serial AFB stains of sputum and pericardial fluid were all negative. Hospital day 3: The initial pericardial fluid culture yielded yeast growth. Hospital day 4: Candida parapsilosis was identified from the pericardial fluid and gram-positive cocci was observed in the pericardial fluid culture. Hospital day 7: AFB stain of pericardial fluid was positive. Hospital day 9: Polymicrobial pathogens were identified subsequently from the initial pericardial fluid culture. Hospital day 10: AFB stain of the pericardial fluid from the operation field was positive. TB cultures of pericardial fluid performed five times were all negative.
AFB, acid fast staining; TB PCR, tuberculosis polymerase chain reaction; INH, isoniazid; RFP, rifampin; EMB, ethambutol; PZA, pyrazinamide; HD, hospital day; MSSA, methicillin susceptible Staphylococcus aureus.
aHD#1 - Culture was requested on hospital day 1.
bPericardial fluid culture requested at hospital day 1 was reported at hospital day 4.
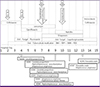
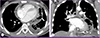
Figure 4
Anteroposterior chest radiograph obtained on day 7 of hospitalization; the cardiac silhouette decreased in size but a newly developed radiolucent lesion around the heart with left costophrenic angle blunting can be seen (arrows).

Figure 5
Chest computed tomography on day 7 of hospitalization: A transverse section image shows pericardial effusion was slightly decreased but multifocal air density and septations in pericardial space (panel A, arrow) were observed. In the coronal section image, subcarinal lymph node within air density (panel B, arrow) appears unchanged, and percutaneous pericardial drainage tube in pericardial space (panel B, arrowhead) is observed.
References
1. Maisch B, Seferović PM, Ristić AD, Erbel R, Rienmüller R, Adler Y, Tomkowski WZ, Thiene G, Yacoub MH. Task Force on the Diagnosis and Management of Pricardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004; 25:587–610.
2. Shiber JR. Purulent pericarditis: Acute infections and chronic complications. Hosp Physician. 2008; 44:9–18.
3. Ferreira dos Santos L, Moreira D, Ribeiro P, Rodrigues B, Correia E, Nunes L, Sequeira M, Albuquerque A, Barros I, Saraiva JP, Santos O. Purulent pericarditis: a rare diagnosis. Rev Port Cardiol. 2013; 32:721–727.

4. Rubin RH, Moellering RC Jr. Clinical, microbiologic and therapeutic aspects of purulent pericarditis. Am J Med. 1975; 59:68–78.

5. Parsons R, Argoud G, Palmer DL. Mixed bacterial infection of the pericardium. South Med J. 1983; 76:1046–1048.

6. Epstein SK, Winslow CJ, Brecher SM, Faling LJ. Polymicrobial bacterial pericarditis after transbronchial needle aspiration. Case report with an investigation on the risk of bacterial contamination during fiberoptic bronchoscopy. Am Rev Respir Dis. 1992; 146:523–525.

7. Lu DC, Chang SC, Chen HC. Polymicrobial bacterial pericarditis with mediastinitis after endotracheal intubation. Diagn Microbiol Infect Dis. 1995; 23:115–118.

8. Sawaya FJ, Sawaya JI, Gharzuddine W, Eid EV, Kanj SS. Cardiac tamponade caused by polymicrobial gram-negative organisms. Int J Infect Dis. 2009; 13:e483–e484.

9. Louw A, Tikly M. Purulent pericarditis due to co-infection with Streptococcus pneumoniae and Mycobacterium tuberculosis in a patient with features of advanced HIV infection. BMC Infect Dis. 2007; 7:12.
10. World Health Organization (WHO). Global tuberculosis report 2013. Accessed 17 May 2014. Available at: http://reliefweb.int/sites/reliefweb.int/files/resources/9789241564656_eng.pdf.
11. Trauter BW, Darouiche RO. Tuberculous pericarditis: optimal diagnosis and management. Clin Infect Dis. 2001; 33:954–961.

12. Stirling P, Faroug R, Amanat S, Ahmed A, Armstrong M, Sharma P, Qamruddin A. False-negative rate of Gram-stain microscopy for diagnosis of septic arthritis: suggestions for improvement. Int J Microbiol. 2014; 2014:830857.

13. Marchiori E, Francisco FA, Zanetti G, Hochhegger B. Lymphobronchial fistula: another complication associated with lymphobronchial tuberculosis in children. Pediatr Radiol. 2013; 43:252–253.

14. Yoon JH, Jung JY, Min JW, Park SY, Jeon YD, Hong HC, Bang JH, Joh JS. Lymphobronchial fistula of tuberculous lymphadenitis in acquired immunodeficiency syndrome. Infect Chemother. 2012; 44:35–39.

15. Bennett JA, Haramati LB. CT of bronchopericardial fistula: an unusual complication of multi drug-resistant tuberculosis in HIV infection. AJR Am J Roentgenol. 2000; 175:819–820.
16. Owens CM, Hamon MD, Graham TR, Wood AJ, Newland AC. Bronchopericardial fistula and pneumopericardium complicating invasive pulmonary aspergillosis. Clin Lab Haematol. 1990; 12:351–354.

17. Dabar G, Daher M, Harmouche C. Bronchopericardial fistula treatment by a metallic stent. Rev Mal Respir. 2013; 30:429–432.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download