Abstract
A 70-year-old man presented with lower back pain and cyanotic changes in his left lower extremity. He was diagnosed with infected aortic aneurysm and infectious spondylitis. He had received intravesical Bacillus Calmette-Guérin (BCG) therapy up to 1 month before the onset of symptoms. The aneurysm was excised and an aorto-biiliac interposition graft was performed. Mycobacterium tuberculosis complex was cultured in the surgical specimens. Real-time polymerase chain reaction (PCR) targeting the senX3-regX3 region, and multiplex PCR using dual-priming oligonucleotide primers targeting the RD1 gene, revealed that the organism isolated was Mycobacterium bovis BCG. The patient took anti-tuberculosis medication for 1 year, and there was no evidence of recurrence at 18 months follow-up.
Intravesical Bacillus Calmette-Guérin (BCG) instillation is one of the most effective treatment options for non-muscle-invasive bladder cancer, and is generally well tolerated [1]. BCG is an attenuated, but live, strain of mycobacteria, belonging to the Mycobacterium tuberculosis complex. It is capable of invasive behavior and occasionally causes systemic or local infections, particularly in immunocompromised hosts [23]. Thus, following intravesical BCG therapy BCG infections including granulomatous prostatitis, epididymo-orchitis, osteomyelitis, psoas abscess, and systemic disseminated infection, have been reported, although such events are uncommon [45]. We report here a case of infected aortic aneurysm caused by Mycobacterium bovis after intravesical BCG instillation.
A 70-year-old man presented with a 1-month history of lower back pain. Three days before admission he had developed cyanotic changes in his left lower extremity, which gradually worsened. Eighteen months previously the patient had been diagnosed with superficial bladder cancer and had undergone transurethral resection of the bladder tumor (TUR-B), followed by intravesical BCG therapy (Onco Tice strain BCG; 12.5 mg BCG injected through a catheter into the bladder every week for 6 weeks). Three months later, another TUR-B was performed because atypical cells were observed in his urine. Histological examination revealed transitional cell carcinoma in situ and he underwent another 3 cycles of intravesicular BCG therapy (13 months, 10 months, and 1 month prior to presentation).
The patient's temperature was 37.5℃ and his other vital signs were stable. Computed tomography of the abdomen revealed an aortic aneurysm involving the abdominal aortic bifurcation and left proximal common iliac artery, with inflammatory soft tissue infiltration in the pre-vertebral space and focal pseudoaneurysm; these had not been seen on a scan performed 17 months earlier (Fig. 1). Magnetic resonance imaging of the lumbar spine revealed minor cortical erosion of the L4 vertebral body and paravertebral phlegmonous and abscess-like soft tissue lesions around the L4 vertebral body (Fig. 2).
Excision of the aneurysm, wide debridement of the psoas abscess, and an in situ aorto-biiliac interposition graft with a rifampicin-soaked Dacron graft were performed. The graft was fully covered with omentum. Surgical exploration confirmed a 3×3 cm cystic lesion in the anterior of the psoas muscle, with a hole leading to the site of suspected spondylitis. Pathologic examination revealed chronic granulomatous inflammation of the vascular wall, but microscopy did not reveal any microorganism. A polymerase chain reaction (PCR) was positive for M. tuberculosis complex, and 10 colonies of this complex were isolated after 31 days of culture of the resected aneurysm specimen.
Two PCR assays were performed to differentiate the pathogen from other members of the M. tuberculosis complex and to identify sub-strains of BCG. Real-time PCR, targeting the 53-bp mycobacterial interspersed repetitive units (MIRUs) of the senX3-regX3 intergenic region (IR), was carried out to differentiate M. bovis BCG and non-BCG M. tuberculosis complex. BCG strains contain only 77-bp mycobacterial interspersed repetitive units (MIRU) within the senX3-regX3 IR, whereas other strains of the M. tuberculosis complex contain both 77-bp and 53-bp MIRUs [67]. In addition, we performed a multiplex PCR assay, using dual-priming oligonucleotide primers targeting the RD1 gene, for simultaneous identification of the M. tuberculosis complex and M. bovis BCG. Both the real-time PCR and the multiplex PCR revealed that the isolated organism was M. bovis BCG.
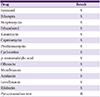
Antibiotic susceptibility testing showed that the organism was resistant to pyrazinamide but susceptible to other drugs (Table 1). Anti-tuberculosis medication with isoniazid, rifampicin, ethambutol, and levofloxacin was started after confirmation of pathologic diagnosis. After 2 months, levofloxacin was discontinued, and isoniazid, rifampicin, and ethambutol were continued for additional 10 months. No signs of recurrent infection were found at 18-month follow-up.
BCG has been used as a live attenuated vaccine of M. bovis against human tuberculosis since 1921 and as immunotherapy for cancer since 1976 [8]. Intravesical BCG instillation therapy has been proven to be safe. According to Lamm et al. [4], the most common complications among 2,602 patients were fever (2.9%), hematuria (1%) and infectious granulomatous, while complications such as prostatitis, pneumonia, hepatitis, osteomyelitis and life-threatening BCG sepsis were rare (<1%). Although intravesical BCG therapy has been widely implemented in Korea, there are only a few case reports of local infectious complications. With regard to systemic infectious complications, there is one case report of granulomatous hepatitis [9] and one of pulmonary complications [10], but in both instances only pathologic evidence suggesting M. bovis infection was presented and the infection was not confirmed microbiologically. We report here a case of a systemic infectious complication involving an infected aortic aneurysm and infectious spondylitis with microbiological confirmation of M. bovis in a patient who had received intravesical BCG therapy.
Infected aneurysm occurring concomitant with infective spondylitis is rare [1112]. To date, fewer than 30 patients with infected aneurysm following intravesical BCG therapy have been reported [1314]. In 2009, Coscas et al. [15] analyzed 21 patients with aneurysm after BCG therapy and reported that the mean interval between completion of BCG therapy and diagnosis of aneurysm was 23 months (range 4-69 months) and the most frequently involved artery was the abdominal aorta (76%), followed by the aortic arch, femoral arteries, popliteal arteries and carotid arteries. In our case, the interval between completion of BCG therapy and diagnosis of infected aneurysm was less than 1 month (17 days) and the multiple infected aneurysm involved the abdominal aortic bifurcation and the left proximal common iliac artery.
Infection of the aorta after instillation of BCG may be due to either hematogenous spread through the vasa vasorum or lymphatic spread through the retroperitoneum. The aorta can also become infected after direct spread of the infection from a psoas abscess or osteomyelitis [1112131415]. In our case, the latter mechanism may well have been responsible because the hole communicated with a psoas abscess, and the infected aortic aneurysm was observed during surgical inspection. Since the risk of metastatic spread may increase significantly when the bladder epithelium is grossly disrupted, BCG should not be instilled within 2 weeks of transurethral resection of bladder cancer, or bladder biopsy. For the same reason, it should not be given if signs or symptoms of macroscopic hematuria or traumatic catheterization remain. Other contraindications are urethral stenosis, active tuberculosis, previously documented BCG sepsis, urinary tract infection, and immunosuppression [16]. In our case, intravesical BCG therapy was started 12 days after the second TUR-B.
When BCG infection is suspected after intravesical BCG therapy, anti-tuberculosis treatment should be promptly initiated. It is important to choose the most appropriate anti-tuberculosis agent but data on the susceptibility of BCG to different anti-tuberculosis agents are limited. As M. bovis has lost the pyrazinamidase gene, all BCG vaccine strains are intrinsically resistant to pyrazinamide [17]. To our knowledge, there are no published data on the anti-tuberculosis susceptibility profile of the BCG Tice strain used for intravesical BCG therapy in Korea. In our case, the M. bovis isolated was susceptible to all the anti-tuberculosis agents tested, except pyrazinamide.
We report here the first case of infected aneurysm with spondylitis following BCG instillation in Korea, and show that the causative species was the mycobacterium, M. bovis BCG. This case emphasizes that, although intravesical BCG therapy is considered safe, systemic complications resulting from vascular infection occasionally occur.
Figures and Tables
Figure 1
Contrast-enhanced computed tomography of the abdomen and lower extremity revealed inflammatory soft tissue infiltration in the prevertebral space and left psoas muscle, with probable abscess formation (A, C, round), focal pseudoaneurysm (B, arrow), and occlusion of the left posterior artery (D, arrow).
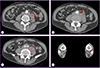
Figure 2
Magnetic resonance imaging of the lumbar spine revealed anterior paravertebral phlegmonous and abscess-like soft tissue lesions at L4 (arrow).
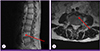
Table 1
Susceptibilities of the isolated Mycobacterium bovis Bacillus Calmette-Guérin (BCG) to anti-tuberculous drugs
References
1. Gontero P, Bohle A, Malmstrom PU, O'Donnell MA, Oderda M, Sylvester R, Witjes F. The role of bacillus Calmette-Guérin in the treatment of non-muscle-invasive bladder cancer. Eur Urol. 2010; 57:410–429.

2. Kwon HJ, Chung BH, Choi BM, Park KU, Kim YK. Severe osteomyelitis as a complication of Tokyo-172 BCG vaccination. J Korean Med Sci. 2012; 27:221–224.

3. Stone MM, Vannier AM, Storch SK, Peterson C, Nitta AT, Zhang Y. Meningitis due to iatrogenic BCG Infection in two immunocompromised children. N Engl J Med. 1995; 333:561–563.

4. Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, Soloway MS, Steg A, Debruyne FM. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992; 147:596–600.

5. Koya MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol. 2006; 175:2004–2010.

6. Lee HR, Kim SY, Chang HE, Song SH, Lee HS, Park KU, Song J, Kim EC. Novel multiplex PCR using dual-priming oligonucleotides for detection and discrimination of the Mycobacterium tuberculosis complex and M. bovis BCG. J Clin Microbiol. 2010; 48:4612–4614.

7. Kim SH, Kim SY, Eun BW, Yoo WJ, Park KU, Choi EH, Kim EC, Lee HJ. BCG osteomyelitis caused by the BCG Tokyo strain and confirmed by molecular method. Vaccine. 2008; 26:4379–4381.

8. Chung JY, Lee ES, Lee WJ, Kim HH, Min KJ, Lee C. Analysis of the immunologic mechanism of intravesical bacillus Calmette-Guerin therapy for superficial bladder tumors: distribution and function of immune cells. J Korean Med Sci. 1993; 8:135–144.

9. Son JI, Chung JP, Paek SS, Lee JI, Kim JH, Moon BS, Lee KS, Lee SI, Moon YM, Park CI, Kim HR, Chung BH. A case of granulomatous hepatitis developed after intravesical BCG instillation for bladder cancer. Korean J Gastroenterol. 2002; 39:375–378.
10. Lee GS, Lee GY, Yoon JC, Na DJ, Jeong SS, Sul CK, Kim SY, Kim JO. Two cases of pulmonary complications following intravesical bacillus Calmette-Gurin immunotherapy in patients with superficial bladder cancer. Tuberc Respir Dis. 1999; 46:869–878.

11. Dahl T, Lange C, Ødegård A, Bergh K, Osen SS, Myhre HO. Ruptured abdominal aortic aneurysm secondary to tuberculous spondylitis. Int Angiol. 2005; 24:98–101.
12. Samadian S, Phillips FM, Deeab D. Mycobacterium bovis vertebral osteomyelitis and discitis with adjacent mycotic abdominal aortic aneurysm caused by intravesical BCG therapy: a case report in an elderly gentleman. Age Ageing. 2013; 42:129–131.

13. Gonzalez OY, Musher DM, Brar I, Furgeson S, Boktour MR, Septimus EJ, Hamill RJ, Graviss EA. Spectrum of bacille Calmette-Guérin (BCG) infection after intravesical BCG immunotherapy. Clin Infect Dis. 2003; 36:140–148.

14. Harding GE, Lawlor DK. Ruptured mycotic abdominal aortic aneurysm secondary to Mycobacterium bovis after intravesical treatment with bacillus Calmette-Guérin. J Vasc Surg. 2007; 46:131–134.

15. Coscas R, Arlet JB, Belhomme D, Fabiani JN, Pouchot J. Multiple mycotic aneurysms due to Mycobacterium bovis after intravesical bacillus Calmette-Guérin therapy. J Vasc Surg. 2009; 50:1185–1190.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download