Abstract
Serratia marcescens causes various diseases. Skin ulcer is one of S. marcescens related diseases, but it is rare clinical syndrome. We experienced a case of skin ulcer caused by S. marcescens in a woman with alcohol induced cirrhosis. After exposure to fresh water while trimming the codfish, she developed deep ulcer on her right hand and bacteremia by S. marcescens. S. marcescens should be considered as a specific etiology of skin infection presenting after fresh water exposure.
S. marcescens belongs to the family Enterobacteriaceae and produces red pigments which develop color change as the colonies age (1). S. marcescens causes not only nosocomial infections but also uncommon community acquired infectious diseases. Some of soft tissue infections that are reported to be caused by S. marcescens are cellulitis and necrotizing fasciitis (2). Skin ulcer caused by S. marcescens is rare clinical syndrome and the portal of entry of community acquired S. marcescens skin infection have not been clearly described in most of the previous reports (3). We experienced a case of skin ulcer caused by S. marcescens in a patient with alcoholic liver disease after having been exposed to fresh water.
A 54 year-old woman who has been a chronic alcohol abuser was admitted due to general weakness and right hand pain. She has worked at a restaurant as a maid. Five days before admission, the tip of her right thumb was pricked by a thorn of an iced codfish while she was trimming using the same subterranean water that the fish was soaked in.
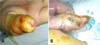
On physical examination, she had blood pressure of 110/70 mmHg, pulse rate of 80/min, respiratory rate of 16 breaths/min, and body temperature of 37.8℃. She was acutely ill looking but showed alert mentality. Spider nevi were observed on her anterior chest. Abdomen was slightly distended, but her liver nor spleen was palpated. At the time of admission, her right hand was swollen up to the wrist and a puncture site was observed at the tip of her right thumb (Fig. 1A), where she felt pain and tenderness was noted. There was no palpable crepitus. Laboratory tests showed the following results: leukocyte count of 4,900/µL with 89.6% neutrophils and 7.4% lymphocytes; hemoglobin, 11.1 g/dL; platelet, 33,000/µL; sodium, 136 mmol/L; potassium, 4.7 mmol/L; chloride, 105 mmol/L; blood urea nitrogen, 102.6 mg/dL; serum creatinine, 2.2 mg/dL; albumin, 2.8 g/dL; total protein, 5.5 g/dL; total bilirubin, 1.8 mg/dL; calcium, 7.2 mg/dL, and phosphorus, 4.9 mg/dL. Radiographs of right hand and upper arm showed soft tissue swelling without gas shadow. Sonographic findings of abdomen showed coarse-echogenic liver, splenomegaly, and ascites, suggestive of alcohol-induced cirrhosis.
Intravenous administration of cefotaxime 2.0 g every 24 hr and ciprofloxacin 200 mg every 12 hr was initiated empirically. On the following day, she had about 200 mL of melena and therefore gastroscopic examination was conducted; it revealed gastric varix and stomach ulcer. On the 3rd day of her admission, her right thumb became deeply ulcerated with undermined margins and palpable fluctuations (Fig. 1B). The pain and swelling increased over the next few days. The swelling and pain had spread from her right hand to her right mid-forearm. A clinical diagnosis of skin ulcer and pyomyositis was made. On the 7th hospital day, incision was made to drain the fluctuant abscess of the right thumb. Pus was drained out from the incised wound. A magnetic resonance imaging scan of the forearm revealed inflammation and an enhancing fluid collection in the forearm muscles. After that day, a fluctuating pocket also became palpable on her right forearm and pus was drained. From the blood and urine culture obtained at admission, S. marcescens was grown and found to be susceptible to cefotetan, ceftriaxone, ciprofloxacin, gentamicin, and trimethoprim/sulfamethoxazole. Gram stain and culture from the wound was not obtained on the 1st day of admission. Pus culture from the forearm on the 7th hospital day was positive for methicillin sensitive Staphylococcus aureus and gram stain was negative. Antibiotics were changed to ciprofloxacin 200 mg every 12 hr and nafcillin 3 g every 6 hr. After two days, she defervesced and her condition gradually improved. She was discharged with oral trimethoprim/sulfamethoxazole therapy.
S. marcescens is commonly found in the environment but not in human fecal flora, thus infections are due to exogenous origins (1). The organism can survive under variable conditions and has been isolated from water, soil, sewage, foodstuffs, and animals (4). S. marcescens is mainly a nosocomial pathogen but causes various community acquired infectious illnesses.
A variety of infections such as pneumonia, bacteremia, urinary tract infection, endocarditis, meningitis, and musculoskeletal system infections are reported to be caused by S. marcescens. S. marcescens has been implicated as the uncommon causes of community acquired soft tissue infections like cellulitis, dermal abscess, and necrotizing fascitis (2). Skin nodules and ulcers may be caused by infectious organisms such as staphylococci, streptococci, mycobacteria, and fungi (5). Fiedman et al. made a report on rapidly progressive skin ulcer and dermal abscess caused by S. marcescens and João et al. reported on painful nodule and ulcers caused by the same bacteria (3, 6). Thus, to the best our knowledge, this will be the third case report that documents skin ulcer caused by S. marcescens.
The most soft tissue infections by S. marcescens have no definitive portal of entry. Fiedman et al. who reported a skin ulcer case suggested a toe web infection as a presumed portal of entry. Curtis et al. experienced a case of necrotizing fascitis caused by S. marcescens after the patient scraped his leg on the rocks in a river while fishing but without a cut in the skin (7). In our case, the patient was pricked by a thorn of a codfish while trimming the fish using subterranean water five days before her symptom development. Given that S. marcescens is widespread in the environment and that skin ulcer developed after the injured thumb was exposed to water, we say that water exposure should be the cause of S. marcescens infection. S. marcescens was not cultured from the wound. It could have been due to the fact that the wound culture was performed 7 days after admission while cefotaxime, which is sensitive to gram negative bacilli, was being administered, which could have disturbed the growth of S. marcescens. However, since S. marcescens was cultured from the blood and urine samples obtained at the time of admission, it could should be regard as the causative organism. S. aureus should be considered as coinfection or super imposed infection. The subterranean water and the thorn of iced codfish exposed to the patient were not cultured. Therefore, the possibility of the fish having been contaminated with S. marcescens cannot be ruled out. The microorganisms that cause soft tissue infections after water exposures are Aeromonas species, Edwardsiella tarda, Erysipelothrix rhusiopathiae, Vibrio vulnificus, and Mycobacterium marinum. This present case and the report by Curtis et al. suggest that fresh water exposure could be the cause of S. marcescens skin infection.
S. marcescens infections occur more commonly in immunocompromised or agranulocytopenic patients (8). Alcohol abuse and chronic liver disease can predispose patients to a variety of infections including pneumonia, tuberculosis, and cellulitis (9). Infectious diseases could occur frequently and more seriously in alcoholic cirrhosis patients. This vulnerability to infections is due to the interference with normal host immune defenses and malnutrition (10).
In summary, this case illustrated that S. marcescens should be considered as a possible cause of soft tissue infection that can develop after fresh water exposure, especially in immunocompromised patients.
Figures and Tables
References
1. Donnenberg MS. Mandell GL, Bennett JE, Dolin R, editors. Enterobacteriaceae. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 2005. 6th ed. Philadelphia: Churchill Livingstone;2567.
2. Liangpunsakul S, Pursell K. Community-acquired necrotizing fasciitis caused by Serratia marcescens: case report and review. Eur J Clin Microbiol Infect Dis. 2001. 20:509–510.

3. Friedman ND, Peterson NB, Sumner WT, Alexander BD. Spontaneous dermal abscesses and ulcers as a result of Serratia marcescens. J Am Acad Dermatol. 2003. 49:S193–S194.

4. Yu VL. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979. 300:887–893.
5. Roth RR, James WD. Microbiology of the skin: resident flora, ecology, infection. J Am Acad Dermatol. 1989. 20:367–390.

6. Joo AM, Serrano PN, Cacho MP, Brtolo EA, Brando FM. Recurrent Serratia marcescens cutaneous infection manifesting as painful nodules and ulcers. J Am Acad Dermatol. 2008. 58:S55–S57.
7. Curtis CE, Chock S, Henderson T, Holman MJ. A fatal case of necrotizing fasciitis caused by Serratia marcescens. Am Surg. 2005. 71:228–230.

8. Bornstein PF, Ditto AM, Noskin GA. Serratia marcescens cellulitis in a patient on hemodialysis. Am J Nephrol. 1992. 12:374–376.

9. MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997. 17:291–315.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download