Abstract
Hematopoietic stem cell transplantation (HSCT) can cause various complications involving lung, liver, intestine and other organs. Chemotherapy, radiation therapy, and graft-versus-host disease (GVHD) may injure the cells in the intestinal mucosa of HSCT recipients. Pneumatosis cystoides intestinalis (PI) is a condition that presence of air in the bowel wall is demonstrated by radiologic or pathologic tests. It is one of the infrequent complications after HSCT and is associated with several medical and surgical conditions. However its pathogenesis and definite etiologic factors are still unknown. Here, we present a case of PI in a HSCT recipient, who was diagnosed of bronchiolitis obliterance accompanied with chronic GVHD and pulmonary disease caused by Mycobacterium abscessus.
Pneumatosis cystoides intestinalis (PI) is defined as gas collection in subserosa or submucosa of the gastrointestinal tract (1, 2). The cause of PI is variable from benign to life-threatening diseases (3). Associated conditions are mechanical, inflammatory, autoimmune, infectious, pulmonary, and drugs including immunosuppressive agents (3). However the pathogenesis is uncertain. Recently, we experienced a case of PI in an allogenic hematopoietic stem cell transplant (HSCT) patient with bronchiolitis obliterans who had received steroid therapy for a long time and had been treated for pulmonary infection caused by nontuberculous Mycobacterium (NTM). Here, we present a case of PI, which is one of the infrequent complications after HSCT and discuss associated factors with PI.
Thirty-two years old woman who complained of mild abdominal pain for 1 day was admitted to the hospital. At the time of admission, her blood pressure was 100/80 mmHg, pulse rate was 119 beats per minute, respiration was 24 per minute, and body temperature was 36.4℃. Physical examination revealed mild tenderness on the whole abdomen but no rebound tenderness was seen. Forty-six months ago, she underwent matched- related bone marrow transplantation from her sister for acute lymphoblastic leukemia. Ten months after HSCT, the patient was diagnosed with bronchiolitis obliterans, and maintained prednisolone (20 mg daily) and tacrolimus (1 mg daily). On 14 months after HSCT, she was diagnosed of pulmonary infection due to Mycobacterium abscessus based on clinical manifestations, three positive sputum cultures and chest CT scan findings (American Thoracic Society guideline) (4). She started cefoxitin, amikacin and clarithromycin for three weeks and then the antibiotics were changed to clarithromycin and levofloxacin for next 24 months. After that the patient was doing well and continued immunesuppresive agents (prednisolone 20-30 mg daily, tacrolimus 1 mg daily) for the treatment of bronchiolitis obliterans. Three months before admission, the patient complained of hoarseness and dyspnea. Laryngoscopic examination revealed whitish plaque on the vocal cord. Chest CT scan showed newly developed multiple cavitary lesions at posterior segment of right upper lung. We considered it as reactivation of previous pulmonary disease and laryngitis caused by M. abscessus. We empirically treated her again with cefoxitin, amikacin and azithromycin for 2 weeks and then administered oral azithromycin and moxifloxacin for maintenance therapy. Vocal cord biopsy revealed only chronic inflammation and no histologic evidence of mycobacterial infection. Although mycobacterial culture from the vocal cord tissue was confirmed negative, M. abscessus was repeatedly isolated from series of sputum cultures. After a month of treatment, follow-up laryngoscopy showed improvement of vocal cord lesion.
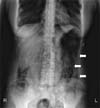
At admission, initial laboratory findings showed white-cell count of 7,540/mm3, with a differential count of 84.7% polymorphonuclear cells, 13.2% lymphocytes, 1.9% monocytes. The hemoglobin level was 14.4 g/dL and the platelet count was 174,000/mm3. The serum creatinine level was 0.65 mg/dL, total bilirubin was 0.91 mg/dL, AST was 28 U/L, ALT was 53 U/L, albumin was 3.55 g/dL, random blood sugar was 226 mg/dL and LDH was 614 IU/L. Urinalysis revealed no specific findings. C-reactive protein was 23.36 mg/dL (normal range, <0.3 mg/dL). Stool culture revealed no microorganism. The result of Clostridium difficile toxin assay was negative. CT scan of the chest showed regression of consolidation and cavity with irregular wall in the right upper lung which was seen 3 months ago. Findings of abdomen X-ray suggested the possibility of intestinal wall emphysema (Fig. 1). CT scan of the abdomen revealed circular collection of gas in the entire large bowel wall and air cyst formation around splenic flexure, which were consistent with the findings of PI (Fig. 2).
She received conservative therapy including bowel rest and parenteral nutritional support. We continued medications for M. abscessus using amikacin, moxifloxacin and azithromycin intravenously. Oral prednisolone was replaced with intravenous methylprednisolone (125 mg daily) and dose of tachrolimus was increased to 2 mg for bronchiolitis obliterans. Gas collection in the large bowel was decreased and her symptoms improved after one month. On the twenty third hospital day, chest X-ray showed newly developed pneumonic consolidation and multiple cavitary lesions in right upper lung. The optical density of serum galactomannan enzyme immunoassay was 7.520 (normal range, <0.5). Later, Aspergillus fumigatus grew on sputum culture. According to the EORTC/MSG criteria, it was considered as probable case of invasive pulmonary aspergillosis. Though the amphotericin B deoxycholate was added immediately, dyspnea worsened and mechanical ventilator was applied. On the twenty ninth hospital day, her condition rapidly deteriorated and she expired due to the progression of multiorgan failure.
PI is characterized by presence of gas in the bowel wall. Many patients are usually asymptomatic, but occasionally they have mild abdominal pain. It usually has been reported in premature infants, and it is less common in older children or adult. Since the first report of PI in 1754 by Duvernoy, many cases of PI have been reported (5). However, PI is an infrequent complication in HSCT patients. In Korea, only one case of PI was reported in HSCT recipient (6). So, this is the second case of PI developed in HSCT patient to be reported in Korea. However, this case has some different points compared with the previous case and these points will be commented on in later part of discussion.
The causes of PI appear to be multifactorial and they can vary from benign to life-threatening conditions (3). Benign conditions associated with PI are pulmonary, collagen vascular, intestinal diseases, medications and transplantation itself (7, 8). Pulmonary diseases including asthma, bronchitis, emphysema, pulmonary fibrosis and cystic fibrosis have associations with PI (3). Systemic diseases such as scleroderma or lupus and intestinal diseases also are important causes of PI (9). Corticosteroid, chemotherapeutic agents, medications that cause bowel distention are also associated with PI (2, 3). Infectious agents including bacteria and cytomegalovirus (CMV) may also contribute to the development of PI (10). There is a theory that gas-forming bacilli enter the submucosa or mucosa and produce gas within the bowel wall (11). Bacterial overgrowth in the gastrointestinal tract from a variety of causes can lead to excessive gas production. Life-threatening causes of PI include intestinal ischemia, mesenteric vascular disease, intestinal obstruction and toxic megacolon (4). There are some reports about fatal cases of PI after HSCT (7, 8, 12). Day DL et al reported 18 cases of PI in HSCT recipients between April 1980 and February 1986 (12). The factors contributing to the PI were pretransplantation chemotherapy and radiotherapy, steroid therapy, infectious colitis, GVHD, and septic shock (12). The development of PI may be caused by the preparation regimens and sequelae of HSCT rather than transplantation itself (12).
Our patient was diagnosed with bronchiolitis obliterans and had to maintain predinisolone (20-30 mg daily) and tacrolimus (1-2 mg daily) over three years. Steroid can cause atrophy of lymphoid aggregates in gastrointestinal tract, which can lead to mucosal defects and allow entrance of intraluminal air into submucosal or subserosal layers. Also, one year after transplant, our patient was diagnosed of pulmonary disease due to M. abscessus and was treated for 2 years. Three months before admission, M. abscessus was reactivated and drugs for NTM were restarted. Although there is no report that medications for NTM are associated with PI, we suggest that long term use of drugs for NTM may have also acted as a potential risk factor for developing PI in our patient. There is one report about PI combined with tuberculous enteritis (13). We did not perform gastrointestinal study because of poor condition of this patient, so we could not exclude the presence of enteritis due to NTM, CMV, or other infectious causes in this case. In our case pp65 CMV antigenemia was negative and tests for C. difficile were also negative. Reactivated NTM might be associated with development of PI in our patient. There was a report in which independent risk factors for NTM included unrelated or mismatched stem cell transplant rather than matched related transplant and existence of bronchiolitis obliterens (14). Our patient was diagnosed as bronchiolitis obliterans and had to maintain immuesuppressive agents for over three years. Bronchiolitis obliterans itself and the use of immunosuppressive agents might be associated with relapsed NTM infection and development of PI in our case.
In most reported cases, PI resolved spontaneously with conservative management and surgical intervention was rarely required. However patients with white-cell count of >12,000/mm3 and/or symptoms of clinical obstruction such as emesis, obstruction and pain, age over 60 years were considered to be candidates for surgical intervention (15). In our case, PI improved with conservative management including bowel rest, parenteral nutrition and antibiotics. However our patient had progressed to multiorgan failure and expired because of combined infectious complications including NTM and invasive pulmonary aspergillosis.
In Korea, the first reported case of PI associated HSCT was presented as acute complication, occurring 2 months after transplant (6). However, in our case PI was developed 4 years after HSCT. PI in our case might be associated not with chemotherapeutic agents or HSCT itself, but with chronic GVHD, long-term use of immunosuppressive agents, NTM infection and NTM treatment. Complications after HSCT are various and sometimes fatal. Also, the management in HSCT recipients is more difficult and complicated than in immunocompetent patients. We hope that this experience can be helpful in evaluation and management of cases of PI in the HSCT recipients in the future.
Figures and Tables
References
1. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis: surgical management and clinical outcome. Ann Surg. 1990. 212:1605.
2. Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA. Pneumatosis intestinalis: a review. Am J Gastroentero. 1995. 190:1747–1758.
3. Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. Am J Roentgenol. 2007. 188:1604–1613.

4. American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997. 156:S1–S25.
5. Koss LG. Abdominal gas cysts (pneumatosis cystoies intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952. 53:523–549.
6. Keam B, Lee JH, Oh MD, Kim I, Yoon SS, Kim BK, Park S. Pneumatosis intestinalis with pneumoperitoneum mimicking intestinal perforation in a patient with myelodysplastic syndrome after hematopoietic stem cell transplantation. Korean J Intern Med. 2007. 22:40–44.

7. Lipton J, Patterson B, Mustard R, Tejpar I, Fyles G, Meharchand J, Messner H. Pneumatosis intestinalis with free air mimicking intestinal perforation in a bone marrow transplant patient. Bone Marrow Transplant. 1994. 14:323–326.
8. Schulenburg A, Herold C, Eisenhuber E, Oberhuber G, Volc-Platzer B, Greinix HT, Reiter E, Keil F, Kalhs P. Pneumatosis [correction of Pneumocystis] cystoides intestinalis with pneumoperitoneum and pneumoretroperitoneum in a patient with extensive chronic graftversus-host disease. Bone Marrow Transplant. 1999. 24:331–333.

9. Rose S, Young MA, Reynolds JC. Gastrointestinal manifestations of scleroderma. Gastroenterol Clin North Am. 1998. 27:563–594.

10. Ho LM, Mosca PJ, Thompson WM. Pneumatosis intestinalis after lung transplant. Abdom Imaging. 2005. 30:598–600.

11. Galandiuk S, Fazio VW. Pneumatosis cystoides intestinalis. A review of the literature. Dis Colon Rect. m. 1986. 29:358–363.
12. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. Am J Roentgenol. 1988. 151:85–87.

13. Pendharker MB, Kadeker SG, Mehta JM, Shah MJ. Pneumatosis cystoids intestinalis associated with pyloric stenosis and tuberculous enteritis. J Assoc Physicians India. 1964. 12:739–743.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download