Abstract
The use of anti-tumor necrosis factor (anti-TNF) agents for rheumatoid arthritis (RA) patients who are refractory to disease-modifying anti-rheumatic drugs is gradually increasing. Etanercept is the first anti-TNF agent to be approved for RA treatment and is also the most widely used. However, aggravation of interstitial lung disease after etanercept treatment in RA patients has been reported recently. We report the first case of recurrent spontaneous pneumothorax with progression of interstitial lung disease after initiating etanercept therapy. The withdrawal of etanercept and a change to adalimumab, a different class of TNF inhibitor, achieved clinical stabilization.
Respiratory manifestations of rheumatoid arthritis (RA) include pleural effusion, pleural thickening, pulmonary fibrosis, bronchiolitis obliterans, bronchiectasis, interstitial pneumonitis, pulmonary hypertension, and necrobiotic nodules. One of the most common pulmonary manifestations of RA is rheumatoid nodules. Spontaneous pneumothorax can be induced either by pulmonary nodules or by the use of anti-rheumatic drugs such as leflunomide and etanercept.
Etanercept is a recombinant-DNA-engineered soluble fusion protein consisting of the extracellular ligand-binding domain of the 75-kD receptor for tumor necrosis factor alpha (TNF-α) coupled to the Fc portion of a monoclonal human antibody. It is administered by subcutaneous injection and acts as a competitive inhibitor of TNF-α. The drug was the first specific anti-cytokine therapy approved for RA.1 Etanercept can provide rapid, significant, and sustained symptom relief in patients with active moderate to severe RA.
However, several side effects to etanercept have been reported. The most common toxicities are injection site reaction, upper respiratory tract infection, and headache. Serious side effects such as reactivation of tuberculosis and demyelinating disease have also been recognized. Indeed, although TNF-α is a pivotal cytokine in the pathophysiology of pulmonary fibrosis,2 a growing number of reports on the development of noninfectious interstitial lung disease (ILD) in patients given etanercept for the treatment of inflammatory joint disease have been published.3,4 Several studies have examined the relationship and pathogenesis of etanercept-induced ILD progression, but the disease mechanism is still unclear.
We recently treated a patient with RA who developed spontaneous pneumothorax with progression of ILD after the initiation of etanercept.
A 43-year-old man with a 2-year history of seropositive RA suffered from suboptimal control of articular manifestations despite medication with nonsteroidal anti-inflammatory drugs (NSAIDs), sulfasalazine, and methotrexate with prednisone. Owing to severe wrist swelling and tenderness in multiple articular joints, his methotrexate dose was gradually increased from 7.5 mg/wk to 15 mg/wk. Hydroxychloroquine was also added to the regimen. Nevertheless, his arthralgia remained uncontrolled, his pain scored on a visual analogue scale (VAS) reached 7 out of 10, and repeated intra-articular steroid injection was needed to control the swelling in both wrists.
In May 2011, after 2 years of such treatment, the patient agreed to start the biological agent etanercept. Although he had no respiratory symptoms, the Mantoux test, chest radiography, and chest computed tomography (CT) were performed before anti-TNF initiation given the high regional prevalence of Mycobacterium tuberculosis in Korea and the patient's 20-year history of smoking. The result of the Mantoux test was positive, so he started taking isoniazid (300 mg/d) for prophylaxis against tuberculosis reactivation. In August 2011, with his methotrexate dose maintained at 15 mg/wk, the patient was started on subcutaneous injection of etanercept 25 mg twice weekly. His articular symptoms dramatically disappeared within 3 months of injection. By November 2011, his VAS pain score decreased from 7 to 2, the number of joints affected decreased from 21 to 5, and his C-reactive protein showed a substantial decrease from 3.36 mg/dL to 0.65 mg/dL. The patient was routinely checked on an outpatient basis every 3 months and showed adequate control of his articular manifestations.
In January 2013, 16 months after initiating etanercept treatment, the patient visited the emergency department with dyspnea and prolonged chest pain. He had no signs of mechanical trauma, and an electrocardiogram showed normal sinus rhythm without any specific abnormality. His chest X-ray revealed right-side pneumothorax (Fig. 1), and thoracostomy was immediately performed. After the procedure, high-resolution computed tomography (HRCT) was taken for further evaluation and showed ground glass attenuation dominant in the basal lungs. Reviewing the HRCT taken in May 2011, we found that his baseline image (Fig. 2A) demonstrated mild subpleural interlobular septal thickening, bronchiolectasis, centrilobular nodules, and ground glass attenuation dominant in the basal lungs. These findings were suggestive of ILD such as usual interstitial pneumonia or nonspecific interstitial pneumonia. After 8 months, the subpleural fibrosis and interlobular septal thickening with bronchiolectasis showed deterioration from the baseline image (Fig. 2B). Through several consultations with the pulmonology department, we concluded that lung biopsy was needed to evaluate the cause of interstitial pneumonia and the development of spontaneous pneumothorax, because the event might have originated from aggravation of lung manifestations of RA, reactivation of pulmonary tuberculosis, or prolonged use of immune-modifying anti-TNF agents.
Two days after admission, primary lung repair and wedge resection were performed via video-assisted thoracic surgery. The biopsied pieces of lung parenchyma were histopathologically evaluated and showed interstitial fibrosis with microscopic honeycomb change that was compatible with usual interstitial pneumonia. The results of nested polymerase chain reaction of Mycobacterium tuberculosis from the biopsied specimen and TB-specific interferon-gamma release assays were negative. Pulmonary tuberculosis was thus ruled out from the possible diagnoses, but we could not exclude the possibility of lung deterioration from the continuous use of etanercept. Clinically, we experience more patients with spontaneous pneumothorax owing to a lung manifestation of RA rather than etanercept-induced lung injury. After 1 week, when the patient's symptoms improved and the chest radiograph showed minimal residual pneumothorax, he was discharged without cessation of etanercept or methotrexate.
Fifteen days after discharge, the patient revisited the hospital complaining of dyspnea. Pneumomediastinum and right pneumothorax were noted in follow-up HRCT images. Both etanercept and methotrexate were stopped, and etanercept was substituted with adalimumab in March 2013 for articular symptom control. Since the cessation of etanercept in February 2013, there has been no recurrence of spontaneous pneumothorax. However, right pleural effusion was observed during the follow-up period. The effusion was analyzed by plain radiographs every month and subsequently developed into loculated effusion suggestive of empyema by August 2013 (Fig. 3). Fortunately, the empyema lesion has consistently decreased in size. The patient now visits our outpatient department for annual chest CT imaging. His latest CT image showed stable ILD without progression (Fig. 2C).
RA is a multi-system disease presenting as destructive inflammatory synovitis and extra-articular manifestations. Disease-modifying anti-rheumatic drugs, such as methotrexate, sulfasalazine, and hydroxychloroquine, have been used to impede disease progression.5 Nevertheless, many patients do not achieve adequate disease control with these drugs. For these patients, newly introduced biological therapies have led to a dramatic improvement in clinical outcomes because they target the key signaling molecules and cells of the immune system. Current biological agents licensed for the treatment of RA encompass a group of TNF-α inhibitors, including infliximab, etanercept, adalimumab, certolizumab, and golimumab.6 Because these agents alter the systemic immune response, there have been concerns over possibly serious systemic side effects. Pulmonary infections have been extensively reviewed in the past. Noninfectious pulmonary complications associated with biological therapies have been less extensively reported since the first case report in 2002.3 However, since then, noninfectious pulmonary complications of the TNF-α inhibitors (infliximab, etanercept, and adalimumab) have been frequently reported.7,8 A recent literature review reported five cases of lung toxicity (including a case of ILD) associated with the relatively newer biological agent golimumab.6 Because anti-TNF agents have been in clinical use for quite some time, we now have a clearer perspective on this adverse phenomenon.
In our case, the fact that etanercept withdrawal stabilized the clinical pulmonary conditions suggests that the occurrence of spontaneous pneumothorax was possibly affected by etanercept treatment. The patient took methotrexate (15 mg/week) for 2 years, and isoniazid was also given prior to the initiation of the anti-TNF agent. Thus, it is reasonable to suggest that these drugs might also be responsible for the patient's clinical course. However, these drugs provoked no respiratory symptoms and so are unlikely to be the main cause of the incidents. Disease activity was well controlled during the etanercept maintenance period; however, after 16 months of treatment, chest tube insertion was needed because of the occurrence of spontaneous pneumothorax. According to a literature review, etanercept-induced ILD is usually confirmed about 2 to 18 months after the first injection dose,6,7,8 similar to the presented case. On the other hand, this case is remarkable because it is the first to report etanercept-induced spontaneous pneumothorax. Interestingly, changing the anti-TNF-α agent from etanercept to adalimumab did not induce further ILD progression, which is assumed to be due to adalimumab's different mode of action. Current biological agents employ two main strategies to neutralize TNF-α: a monoclonal IgG antibody and a soluble TNF-α receptor. Adalimumab is a complete monoclonal human IgG1 anti-TNF-α antibody, whereas etanercept acts as a soluble TNF-α receptor blocker. This difference in their biological mechanisms can explain different clinical effects in our patient.
The overexpression of membrane-bound TNF-α produces spontaneous tissue inflammation, and anti-TNFs are reasonable therapeutic options under ubiquitous conditions. However, these agents seem to have various effects in diverse disease spectrums. For example, the efficacy of various anti-TNFs has been studied in multiple pulmonary diseases such as asthma and COPD, and they have shown varying levels of efficacy in preclinical studies. No anti-TNF has been approved for any of these indications owing to their lack of consistent efficacy and the risk of side effects in clinical trials.9 The hypothesis that anti-TNFs may be beneficial for pulmonary diseases is based on the fact that TNF-α and its mRNA have been detected in surgical pathology specimens of patients with idiopathic pulmonary fibrosis, and blockade of TNF-α has been found to reduce pulmonary fibrosis in vitro. However, anti-TNF agents such as infliximab were shown to be ineffective in a recent randomized prospective trial. To explain this phenomenon, Allen et al. proposed that ILD is associated with a Th2 immune response pattern,10 and the inhibition of TNF-α may alter the Th1/Th2 balance, inducing pro-fibrotic Th2 activation and leading to an exacerbation of interstitial lung involvement. However, since the supporting data are scarce, further studies must be carried out to define this mechanism.
Etanercept treatment in RA patients who show ILD on the baseline chest radiograph may lead to adverse immune system responses. Routine chest radiographs and pulmonary function tests including carbon monoxide diffusion before and after the administration of anti-TNFs are recommended when possible. As in the present case, the cornerstone of treatment for drug-induced lung injury is withdrawal of the drug. Other concomitant drugs such as isoniazid or methotrexate may also give rise to ILD progression. However, because we know that etanercept has the potential to provoke ongoing ILD progression, it is wise to withhold the anti-TNF agent and follow up for clinical stabilization.
We report our experience of using adalimumab as an alternative anti-TNF agent for symptomatic control of an RA patient with etanercept-induced ILD. We believe that adalimumab and other biologics with different mechanisms of action from etanercept are options for the treatment of RA. Additional clinical studies are needed to verify this conclusion.
In summary, the present case suggests that RA patients with underlying interstitial lung disease may suffer from etanercept-induced ILD progression and recurrent pneumothorax. Withholding the drug helps to induce clinical stabilization. Pulmonary toxicities must be considered when using anti-TNF agents in RA patients. The use of other biologics such as adalimumab is a safe and effective option when a biological agent is needed for RA management in etanercept-induced ILD patients.
Figures and Tables
FIG. 1
Chest radiograph showing an absence of lung markings and a pleural line in the right chest, suggestive of spontaneous pneumothorax.
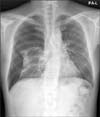
FIG. 2
(A) Baseline high-resolution computed tomography (HRCT) image of the chest. Mild subpleural interlobular septal thickening with ground glass attenuation is seen in both lungs. (B) HRCT image just after the initial onset of pneumothorax. Subpleural fibrosis and bronchiectasis worsened with a definite right-sided pneumothorax. (C) Follow-up HRCT on the same level of the chest. The image was taken after cessation of etanercept and 3 months after medication with adalimumab. One year after etanercept cessation, interstitial fibrosis is still noted but is relatively stable in its degree and extent.

References
1. Jarvis B, Faulds D. Etanercept: a review of its use in rheumatoid arthritis. Drugs. 1999; 57:945–966.
2. Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J. 1994; 7:515–518.

3. Peno-Green L, Lluberas G, Kingsley T, Brantley S. Lung injury linked to etanercept therapy. Chest. 2002; 122:1858–1860.

4. Hagiwara K, Sato T, Takagi-Kobayashi S, Hasegawa S, Shigihara N, Akiyama O. Acute exacerbation of preexisting interstitial lung disease after administration of etanercept for rheumatoid arthritis. J Rheumatol. 2007; 34:1151–1154.
5. Fries JF, Williams CA, Morfeld D, Singh G, Sibley J. Reduction in long-term disability in patients with rheumatoid arthritis by disease-modifying antirheumatic drug-based treatment strategies. Arthritis Rheum. 1996; 39:616–622.

6. Hadjinicolaou AV, Nisar MK, Bhagat S, Parfrey H, Chilvers ER, Ostör AJ. Non-infectious pulmonary complications of newer biological agents for rheumatic diseases--a systematic literature review. Rheumatology (Oxford). 2011; 50:2297–2305.

7. Khasnis AA, Calabrese LH. Tumor necrosis factor inhibitors and lung disease: a paradox of efficacy and risk. Semin Arthritis Rheum. 2010; 40:147–163.

8. Thavarajah K, Wu P, Rhew EJ, Yeldandi AK, Kamp DW. Pulmonary complications of tumor necrosis factor-targeted therapy. Respir Med. 2009; 103:661–669.

9. Morjaria JB, Chauhan AJ, Babu KS, Polosa R, Davies DE, Holgate ST. The role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trial. Thorax. 2008; 63:584–591.

10. Allen JT, Spiteri MA. Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir Res. 2002; 3:13.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download