INTRODUCTION
Gasotransmitters are small molecules of endogenous gases and have important physiological functions. The current known gasotransmitters include nitric oxide (NO), carbon monoxide, hydrogen sulfide (H2S), and others.1-3 It is well known that NO has an inhibitory action in the gastrointestinal (GI) tract. NO is produced by NO synthase (NOS) and plays specific roles in the modulation of contractile functions in GI smooth muscle.4,5
H2S is the most recently identified gaseous mediator. The physiological and pathological functions of H2S have recently attracted intense research interest. Much of this interest stems from the discovery of endogenous H2S generation by the pyridoxal-5'-phosphate-dependent enzymes cystathionine beta-synthase and cystathionine gamma-lyase in mammalian tissues.6,7 In general, H2S has inhibitory effects in the GI tract. H2S results in relaxation of isolated ileal muscle strips8 and inhibits the motor patterns in the colon and jejunum of various species.9
The motility of the GI tract is generated by periodic electrical activity called slow waves, and it is widely accepted that the slow waves originate from the interstitial cells of Cajal (ICC) and are propagated to nearby muscle cells via the gap junction.10 Because of the crucial role of ICC on GI motility, ICC can be a regulatory target for many hormones, neurotransmitters, and various endogenous compounds for modulating GI motility.
The interaction between NO and H2S is an interesting area of study. Many reports have suggested that the effect of H2S is, at least partially, via the activation of NOS.11-13 Also, it was shown that NO donors enhance H2S production and increase cystathionine gamma-lyase expression in cultured smooth muscle cells.14 These reports suggest that H2S and NO may interact with each other.
MATERIALS AND METHODS
1. Animal and tissue preparation
Balb/c mice (3-7 days old) of either sex were anesthetized with diethyl ether and sacrificed by cervical dislocation. All animals were treated ethically according to the guiding principles for the care and use of animals in the field of physiological sciences approved by the Institutional Animal Use and Care Committee at Chosun University College of Medicine. The small intestine was excised 1 cm below the pyloric ring to the cecum and opened along the mesenteric border. The luminal contents were washed away with Krebs-Ringer bicarbonate solution.
2. ICC culture
The isolated tissue was pinned to the base of a Slygard dish, and the mucosa was removed by sharp dissection. Small strips of intestinal muscle were equilibrated in calcium-free Hank's solution with the following constituents (in mM): KCl, 5.36; NaCl, 125; NaOH, 0.336; Na2HCO3, 0.44; glucose, 10; sucrose, 2.9; and HEPES, 11. The pH was adjusted to 7.4 with Tris for 30 minutes. Cells were dispersed by incubating for 15 minutes at 37℃ in an enzymatic solution containing collagenase (1.3 mg/ml; Worthington Biochemicals, Lakewood, NJ, USA), bovine serum albumin (2 mg/ml; Sigma, St. Louis, MO, USA), trypsin inhibitor (2 mg/ml; Sigma), and ATP (0.27 mg/ml). The cells were finely chopped and placed onto sterile glass coverslips coated with poly-L lysine in 35-mm culture dishes and incubated at 37℃ in a 95% O2 and 5% CO2 incubator in smooth muscle growth medium (Cambrex Bio Science, Walkersville, MD, USA) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, USA) and murine stem cell factor (5 ng/ml; Sigma).
3. Patch-clamp experiments
The whole-cell configuration of the patch-clamp technique was used to record membrane currents (voltage clamp) and membrane potentials (current clamp) from the cultured ICC. Currents or potentials were amplified by using an Axopatch 1-D (Axon Instruments, Foster City, CA, USA). Command pulse was applied by using an IBM-compatible personal computer and pClamp software (version 9.2; Axon Instruments). The data were filtered at 5 KHz. All experiments were carried out at 30℃.
4. Solutions and drugs
The cells were bathed in a standard solution containing the following (in mM): KCl, 5; NaCl, 135; CaCl2, 2; glucose, 10; MgCl2, 1.2; and HEPES, 10. The standard solution was adjusted to pH 7.4 with Tris. The pipette solution contained the following (in mM): K-aspartate, 120; KCl, 20; MgCl2, 5; K2ATP, 2.7; Na2GTP, 0.1; creatine phosphate disodium, 2.5; EGTA, 0.1; and HEPES, 5. The solution was adjusted to pH 7.4 with Tris.
All drugs in this study were purchased from Sigma Chemical Co. and were dissolved in appropriate solvent as mentioned in the product information. NaHS was dissolved in water and was used fresh in every experiment.
5. Measurement of the intracellular Ca2+ concentration
Changes in the intracellular Ca2+ concentration ([Ca2+]i) were monitored by using fluo-3/AM, which was initially dissolved in dimethyl sulfoxide and stored at -20℃. The cultured ICC on coverslips (25 mm) were rinsed twice with a bath solution. The coverslips were then incubated in the bath solution containing 1 µM fluo-4 with 5% CO2 at 37℃ for 5 min, rinsed 2 more times with the bath solution, mounted on a perfusion chamber, and scanned every 0.4 s with a Nikon Eclipse TE200 inverted microscope equipped with a Perkin-Elmer Ultraview confocal scanner and a Hamamatsu Orca ER 12-bit CCD camera (×200). Fluorescence was excited at a wavelength of 488 nm, and emitted light was observed at 515 nm. During scanning of the Ca2+ imaging, the temperature of the perfusion chamber containing the cultured ICC was kept at 30℃. The variations of intracellular Ca2+ fluorescence emission intensity were expressed as F1/F0, where F0 is the intensity of the first imaging.
RESULTS
1. Effect of NO+H2S on pacemaker potentials generated by ICC
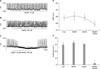
To investigate the effect of NO+H2S, we performed electrophysiological recording from cultured ICC under the current clamp mode (I=0), in which spontaneous depolarization (pacemaker potentials) was generated by ICC. The resting membrane potential was -61±2 mV and the amplitude of the pacemaker potential was 29.5±4 mV. Treatment with a low concentration of (±)-S-nitroso-N-acetylpenicillamine (SNAP; an NO donor; 10 µM) did not influence pacemaker potentials in ICC according to the membrane potentials (Fig. 1A); 100 µM NaHS (a donor of H2S) also did not have an effect (Fig. 1B). However, co-treatment with SNAP (10 µM)+NaHS (100 µM) produced membrane hyperpolarization and decreased the amplitude of the pacemaker potentials (Fig. 1C). In the presence of SNAP and NaHS, the resting membrane was hyperpolarized to -64.8±1 mV (n=3; Fig. 1D) and the amplitude of the pacemaker potentials decreased to 2.1±1.1 mV (n=3; Fig. 1E).
2. Effect of NO+H2S on pacemaker currents generated by ICC
To examine the role of NO+H2S on pacemaker currents in ICC, we tested under a voltage clamp at a holding potential of -70 mV. In the control condition, ICC generated pacemaker currents. Treatment with a high concentration of SNAP (100 µM) or NaHS (1 mM) decreased both the frequency and the amplitude of the pacemaker currents and the resting currents were increased in the outward direction (Fig. 2A, B). The application of a low concentration of SNAP (10 µM) or NaHS (100 µM), however, did not show any influence on pacemaker currents in ICC (Fig. 2C, D). However, co-treatment with a low concentration of SNAP (10 µM)+NaHS (100 µM) inhibited the frequency and amplitude similar to the effect of a high concentration of SNAP or NaHS on pacemaker currents in ICC (Fig. 2E). The value obtained after co-treatment with low concentrations of SNAP and NaHS was significantly different from that obtained after single treatment with low concentrations of SNAP or NaHS (n=3; Fig. 2F-H).
3. Involvement of ATP-sensitive K+ channels and cyclic GMP on NO+H2S-induced effects on pacemaker currents generated by ICC
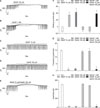
To determine the role of ATP-sensitive K+ (KATP) channels or cyclic guanosine monophosphate (cGMP) on NO+H2S-induced action on pacemaker currents, we used glibenclamide, an inhibitor of KATP channels, and ODQ, an inhibitor of guanylate cyclase. Treatment with ODQ (10 µM) or glibenclamide (10 µM) alone had no effect on pacemaker currents, but pre-treatment with ODQ or glibenclamide completely blocked the SNAP (10 µM)+NaHS (100 µM)-induced effects on ICC (Fig. 3A, B). The value obtained after co-treatment with a low concentration of SNAP and NaHS in the presence of ODQ or glibenclamide was significantly different from that obtained in the absence of ODQ or glibenclamide (n=3; Fig. 3C-E).
4. Inhibition of intracellular Ca2+ oscillation by NO+H2S
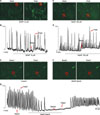
To investigate the role of intracellular Ca2+ ([Ca2+]i) in the NO+H2S-induced action on pacemaker currents, we examined the effect of SNAP (10 µM) + NaHS (100 µM) on [Ca2+]i oscillations in ICC. In this study, we measured spontaneous [Ca2+]i oscillations of ICC, which are connected with cell clusters. Spontaneous [Ca2+]i oscillations were observed in many ICC that were loaded with fluo4-AM. Treatment with SNAP (10 µM) or NaHS (100 µM) did not show any influence on spontaneous [Ca2+]i oscillations of ICC (n=4; Fig. 4A, C). However, in the presence of co-application of SNAP (10 µM)+NaHS (100 µM), the spontaneous [Ca2+]i oscillations of ICC rapidly declined (n=4; Fig. 4E, F). The time series data are shown in Fig. 4B, D, and G.
DISCUSSION
The main objective of this study was to investigate the interaction between H2S and NO in ICC. By electrophysiological study with cultured ICC, we found that 100 µM NaHS or 10 µM SNAP alone had a negligible effect in ICC, which is consistent with our previous report.15,16 However, unexpectedly, when these two donors were mixed together, we saw a marked inhibition of pacemaker currents in ICC. Because stimulating endogenous NO production with L-arginine and exogenous application of NO donors produced similar effects, the possibility that the effect resulted merely from the chemical reaction of the two donors can be excluded.
In our previous reports, we showed that 100 µM SNAP or 1 mM NaHS inhibits pacemaker activity in ICC.15,16 However, to study the interaction of NaHS and SNAP, we needed to find a concentration of NO or NaHS that had no action on ICC; we found that 100 µM NaHS or 10 µM SNAP alone had no effect. It was reported previously that the mixture of NO donors and H2S potentiated the relaxation effect of the NO donors in aortic rings in vitro.17 Also, many reports have suggested that NO donors stimulate H2S production14 an that H2S enhances the activation of NOS.11-13 These reports indicate that H2S and NO can influence the production of each other. Therefore, H2S and NO interact with each other in a number of ways.18 In this study, we also showed that H2S and NO interacted with each other to inhibit the pacemaker activity in ICC.
Our next finding was that the concentrations of NaHS and NO that had no effect on pacemaker activity alone could enhance the inhibitory action by co-treatment in ICC. To determine this, we focused on KATP channels. In our previous report, we showed the existence of KATP channels in ICC with pinacidil, a KATP channel opener,19 and the localization of KATP channels Kir 6.2 and SUR 2B in cultured ICC.20 Also, we reported that SNAP inhibited pacemaker currents that were blocked by an inhibitor of KATP channels in ICC.16 However, we found that glibenclamide could not block the NaHS-induced effect.15 In this study, we found that glibenclamide blocked the effects in ICC induced by NO+H2S. These findings suggest that the enhanced effect of H2S+NO on ICC is by stimulating NO action by H2S. Furthermore, the major second messenger of NO is cGMP by activation of guanylate cyclase in various cells.21 In ICC in particular, some reports have shown that NO inhibits electrical activity by cGMP regulation.16,22 In this study, we found that a guanylate cyclase inhibitor blocked the NO+H2S-induced effects in ICC. This result supports our suggestion that the effect of NO+H2S on ICC is via the stimulation of NO action by H2S.
To understand how NO+H2S inhibits pacemaker activity in ICC, we checked [Ca2+]i by using live cell imaging. It is well known that the periodic pacemaker activity of ICC is dependent on [Ca2+]i oscillation, and that this pacemaker mechanism is initiated by release of Ca2+ from the endoplasmic reticulum through the inositol triphosphate receptor and is followed by re-uptake of Ca2+ into the mitochondria.23,24 Furthermore, many reports have shown that the opening of KATP channels decreases [Ca2+]i levels by inhibiting external Ca2+ influx or release from intracellular store.25,26 If the NO+H2S-induced inhibitory action on ICC is via an enhancement of NO, this mixture should inhibit the [Ca2+]i oscillation. In fact, we found that NO+H2S inhibited the [Ca2+]i oscillation. Interestingly, our previous report showed that FCCP, an inhibitor of mitochondrial Ca2+ uptake, blocked the pacemaker activity of ICC, similar to the action induced by a high concentration of NaHS.15 Namely, in ICC, NO may stimulate KATP channels and then decrease the [Ca2+]i, but NaHS blocks mitochondrial Ca2+ uptake.
In conclusion, the results of the present study indicate that H2S and NO interact in ICC and this interaction results in the inhibition of pacemaker activity. Also, the interaction of H2S and NO is via the stimulation or enhancement of NO. However, further study is needed to understand whether H2S stimulates NO production or increases NO efficacy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download