Abstract
Figures and Tables
Fig. 1
Posterior and mediolateral views of the left humerus showing the parameters used. VDH, vertical head diameter; UEB, upper epiphyseal breadth; TDUS, transverse diameter at the upper half of the shaft; TDMS, transverse diameter at the middle of the shaft; TDLS, transverse diameter at the lower half of shaft; EB, epicondylar breadth; ML, maximum length; CH, circumference of the head; USC, upper shaft circumference; MSC, mid-shaft circumference; LSC, lower shaft circumference.
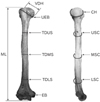
Table 1
Definitions of humeral parameters measured

Table 2
Mean values of variables of humerus in males and females (Students t test)

Values are presented as mean±SEM. ML, maximum length; VDH, vertical head diameter; CH, circumference of the head; TDMS, transverse diameter at the middle of the shaft; TDUS, transverse diameter at the upper half of the shaft; TDLS, transverse diameter at the lower half of shaft; USC, upper shaft circumference; MSC, mid-shaft circumference; LSC, lower shaft circumference; UEB, upper epiphyseal breadth; EB, epicondylar breadth.
Table 3
Demarking points separating males from females, sexual dimorphism ratio, F ratio, and statistical significance in KwaZulu-Natal study population

ML, maximum length; VDH, vertical head diameter; CH, circumference of the head; TDMS, transverse diameter at the middle of the shaft; TDUS, transverse diameter at the upper half of the shaft; TDLS, transverse diameter at the lower half of shaft; USC, upper shaft circumference; MSC, mid-shaft circumference; LSC, lower shaft circumference; UEB, upper epiphyseal breadth; EB, epicondylar breadth.
Table 4
Summary of stepwise discriminant function analyses, unstandardized discriminant function coefficients, and the sectioning points

Table 5
Classification accuracy on humeral dimension in KwaZulu-Natal population

Values are presented as percentage. ML, maximum length; VDH, vertical head diameter; CH, circumference of the head; TDMS, transverse diameter at the middle of the shaft; TDUS, transverse diameter at the upper half of the shaft; TDLS, transverse diameter at the lower half of shaft; USC, upper shaft circumference; MSC, mid-shaft circumference; LSC, lower shaft circumference; UEB, upper epiphyseal breadth; EB, epicondylar breadth.
Table 6
Variables selected by the stepwise discriminant function analysis, the best discriminatory variable for group assessment, and the percentage of accuracy for several different populations

| Study | Population | No. | Variables selected (stepwise) | Accuracy (%) | Best variable | Accuracy (%) |
|---|---|---|---|---|---|---|
| Steyn and Işcan [10] | South Africans whites (Dart and Pretoria) | 104 | EB/VDH | 92.5 | EB | 94.7 |
| Steyn and Işcan [10] | South Africans blacks (Dart and Pretoria) | 88 | VDH/ML | 93.1 | VDH | 96.0 |
| Işcan et al. [32] | Chinese | 87 | ML/VDH/EB/MSC | 86.6 | VDH | 80.5 |
| Işcan et al. [32] | Japanese | 90 | EB/VDH/MMSD/MSC | 92.4 | EB | 89.9 |
| Işcan et al. [32] | Thais | 104 | EB/VDH/MMSD | 97.1 | EB | 93.3 |
| Lee et al. [33] | Koreans | 175 | ML/VDH | 87 | VDH | 87.0 |
| Ríos Frutos [34] | Guatemalans | 118 | MDH/MMSD/EB | 98.5 | MDH | 95.5 |
| Mall et al. [9] | Germans | 143 | ML/VDH/EB | 93.2 | VDH | 90.4 |
| Kranioti and Michalodimitrakis [3] | Cretans | 178 | ML/VDH/MMSD/EB | 92.9 | VDH | 89.9 |
| Present study (2017) | KwaZulu-Natal’s | 211 | VDH/ML/TDLS/MSC | 87.7 | VDH | 82.5 |
Table 7
Comparison of demarking points with other studies

| Study | Population | ML | VDH | MSC |
|---|---|---|---|---|
| Işcan et al. [32] | Chinese | F<298.65<M | F<42.30<M | F<61.10<M |
| Işcan et al. [32] | Japanese | F<287.15<M | F<41.60<M | F<61.40<M |
| Işcan et al. [32] | Thai | F<289.75<M | F<41.20<M | F<57.15<M |
| Lee et al. [33] | Koreans | F<289.50<M | F<42.70<M | - |
| Steyn and Işcan [10] | South African whites (Dart and Pretoria) | F<322.20<M | F<46.04<M | - |
| Steyn and Işcan [10] | South African blacks (Dart and Pretoria) | F<311.35<M | F<40.74<M | - |
| Kranioti and Michalodimitrakis [3] | Cretans | F<307.39<M | F<43.79<M | F<62.03<M |
| Present study (2017) | KwaZulu-Natal's | F<307.52<M | F<39.95<M | F<60.92< M |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download