Abstract
Percutaneous transvenous mitral annuloplasty (PTMA) has evolved as a latest procedure for the treatment of functional mitral regurgitation. It reduces mitral valve annulus (MVA) size and increases valve leaflet coaptation via compression of coronary sinus (CS). Anatomical considerations for this procedure were elucidated in the present study. In 40 formalin fixed adult cadaveric human hearts, relation of the venous channel formed by CS and great cardiac vein (GCV) to MVA and the adjacent arteries was described, at 6 points by making longitudinal sections perpendicular to the plane of MVA, numbered 1–6 starting from CS ostium. CS/GCV formed a semicircular venous channel on the atrial side of MVA. Based on the distance of CS/GCV from MVA, two patterns were identified. In 37 hearts, the venous channel at point 2 was widely separated from the MVA compared to the two ends and in three hearts a nonconsistent pattern was observed. GCV crossed circumflex artery superficially. GCV or CS crossed the left marginal artery and ventricular branches of circumflex artery superficially in 17 and 23 hearts, respectively. As the venous channel was related more to the left atrial wall, PTMA devices probably exert an indirect traction on MVA. The arteries crossing deep to the venous channel may be compressed by PTMA device leading to myocardial ischemia. Knowledge of the spatial relations of MVA and a preoperative and postoperative angiogram may help to reduce such complications during PTMA.
Functional mitral regurgitation (FMR) is observed clinically as a complication of myocardial infarction [1] and dilated cardiomyopathy [2]. It occurs as a result of reduced coaptation between the valve leaflets which normally ensure mitral valve competence [3]. There has been an increase in the incidence of FMR due to an improvement in the long term survival after acute myocardial infarction [4]. Treatment modalities for FMR include coronary artery bypass grafting, mitral valve ring annuloplasty, and mitral valve replacement [56]. High failure rates and concomitant medical conditions may limit the use of these procedures in some patients. This has led researchers to develop percutaneous transvenous mitral annuloplasty (PTMA) devices which reduce the annular size by compressing the coronary sinus (CS). Results of preliminary data from animal [789] and human [10] studies have been promising. The anatomical basis of this technique is that the CS and the distal part of the great cardiac vein (GCV) form a venous channel which travels parallel to the posterior aspect of the mitral valve annulus (MVA) circumferentially [11]. However, the safety and efficacy of the procedure has remained suboptimal due to the CS lying on the left atrial wall for almost its entire course and branches of coronary arteries running between CS/GCV and MVA, thereby producing an inherent risk of compression by PTMA devices [12]. A thorough knowledge of the spatial relationship of CS/GCV venous channel and MVA along with the accompanying arteries would help interventional cardiologists and cardiothoracic surgeons to optimize the safety and efficacy of the procedure.
The study was conducted on 40 formalin fixed adult cadaveric human hearts procured from north Indian adults (age, 24–65 years), out of which 37 were male and three were female. Hearts with congenital anomalies, gross pathology or with history of previous cardiac surgeries were excluded from the study.
The cavity of the left atrium was opened by an incision across the two lower pulmonary veins. Longitudinal sections were made along the free circumference of the MVA between two points. The first point was marked at the CS ostium and the second point where GCV took a bend from the anterior inter-ventricular groove to the atrio-ventricular groove. This part of MVA circumference was divided into six segments of equal length thus creating six points numbered 1–6, starting from CS ostium which was numbered point 1 and ending at point 6 which was located at the acute bend of GCV. At each of the 6 points, the CS or GCV was studied in relation to the left circumflex coronary artery and MVA. The sections were made in each region perpendicular to the plane of MVA. The distance between of the endocardium inferior to the MVA and the nearest endothelial lining of the wall of the veins was measured at each of the 6 points (Fig. 1). The distances were measured by digital vernier callipers (Effem Technologies, New Delhi, India).
The range, mean and standard deviation of all the quantitative data was calculated. The variation in the distance between the CS/GCV and the MVA at the 6 points was analysed by a one-way ANOVA. After this, Duncan multiple range test was performed to find variations among the individual points. The data was analysed using SPSS software version 22.0.0 (IBM Co., Armonk, NY, USA).
CS and GCV were found to form a semi-circular venous channel on the atrial side of MVA. At point 1 to 4, CS was related to MVA, while GCV was related to the annulus at point 5 and 6 (Fig. 2). The distance between CS/GCV and MVA are provided in Table 1.
Two patterns were identified based on the distance between the venous channel and MVA:
(1) In 37 hearts (92.5%), the venous channel at point 2 was widely separated from the MVA compared with the two ends. The closest point was at point 6. The mean distances were 9.34 mm, 10.74 mm, 9.55 mm, 9.17 mm, 6.92 mm, and 6.02 mm at point 1, 2, 3, 4, 5, and 6, respectively. Distances at point 1, 2, 3, and 4 were significantly longer than the distances at point 5 and 6 (P<0.01).
(2) In three hearts (7.5%), a non-consistent pattern was observed. The mean distances were 10.10 mm, 11.11 mm, 10.54 mm, 10.52 mm, 10.34 mm, and 10.46 mm at point 1, 2, 3, 4, 5, and 6, respectively.
The GCV was found to have a triple relation with the left circumflex artery. At first, it was inferior to the artery then crossing the artery superficially, ran superior to it (Fig. 3). In 17 hearts (42.5%), the GCV crossed the left marginal artery superficially (Fig. 4). The CS travelled superior to the left circumflex artery. In 23 hearts (57.5%), ventricular branches of the circumflex artery passed deep to the CS/GCV to reach the left ventricle (Fig. 5).
The interest in studying the morphology of the coronary venous system has seen an escalation in the recent past as it is increasingly being used for various cardiologic interventions. Notable among these are CS catheterisation for metabolic studies of the heart, and measuring CS substrate concentration for assessing myocardial use of substrates and oxygen, measuring CS temperature to determine hypothermic protection during retrograde cardioplegia [13], left ventricular or biventricular pacing in patients with severe heart failure [14] and PTMA. PTMA has emerged as the latest innovation for the treatment of FMR which requires the delivery of an annuloplasty device via CS to reduce the annular dimensions of the mitral valve. The present study elucidated the morphology of CS/GCV with relation to MVA and the neighbouring arteries, which would serve as the anatomical prerequisite for successfully performing PTMA.
In the present study, CS and GCV were found to form a semi-circular venous channel around MVA (more on the left atrial side). Based on the distance between the MVA and the CS/GCV, two patterns were identified. In 92.5% hearts, the venous channel at point 2 was widely separated from the MVA compared with the two ends while a non-consistent pattern was observed in a minority of cases (7.5%). This was similar to the findings of El-Maasarany et al. [15] who reported, other than the patterns observed in the present study, a third pattern in seven hearts where CS was widely separated from the annulus proximally, at its ostium and became closer distally towards the GCV. Such a pattern was not observed in the present study [15].
Yamanouchi et al. [16] studied fifty hearts where the posterolateral part of the MVA was divided perpendicularly into five equal sections. The distance from the ventricular endocardium under MVA to the nearest wall of CS was measured in each cross-section and was reported as 9.7±2.3, 10.9±3.3, 10.2±3.6, 9.2±3.4, and 8.2±2.9 mm at 36°, 72°, 108°, 144°, and 180°, respectively. They reported the distance at 72° and 108° to be significantly longer than at 144° and 180° [16].
Maselli et al. [12], studied the distance of the CS to the MVA and surrounding structures, in 61 excised cadaveric human hearts. Maximal distance from the CS to the MVA was reported to be up to 19 mm (mean, 9.7±3.2 mm). They postulated that the PTMA devices probably shrink the MVA only by an indirect traction mediated by the left atrial wall due to a significant distance of the CS from the MVA [12]. The maximum distance in the present study was 11.68 mm which is much less than maximum distance reported by Maselli et al. [12]. As the venous channel was related more to the left atrial wall than to the circumference of MVA, the hypothesis of indirect traction by PTMA devices cannot be ruled out.
Inherent genetic variations between the populations studied could account for the differences in the results as compared to the previous authors.
In the present study, GCV crossed the circumflex artery superficially in all the hearts studied. In 17 hearts, the GCV crossed the left marginal artery superficially and in 23 hearts, ventricular branches of the circumflex artery passed deep to the CS to reach the left ventricle. Thus, a PTMA device in situ might compress these arteries leading to myocardial ischemia.
Previous studies have reported that in 45%–95% patients, CS crossed over the circumflex artery or the marginal artery [17]. Harnek et al. [18] emphasized the need of a preoperative imaging in cases where such a crossing occurs.
A preoperative and a postoperative angiogram can give valuable information about arterial compression and should be carried out after PTMA in every patient.
Clinical trials on PTMA devices has shown to reduce mitral regurgitation, to reverse left ventricular remodelling [10], and cause a sustained favourable geometric modification of the MVA [19] but the mortality rate of the procedure is high and the risk/benefit ratio still remains suboptimal [20].
The distance of the semicircular venous channel (formed by CS and GCV) to the MVA and their relation to the accompanying arteries described in the present study will help interventional cardiologists and cardiothoracic surgeons to develop better PTMA devices making the risk/benefit ratio more favourable. The data presented in this study may be used in conjunction with preoperative and postoperative imaging modalities, in order to tailor the PTMA device and procedure according to the need of a patient individually.
Figures and Tables
Fig. 1
A longitudinal section of the heart showing coronary sinus related superiorly to the mitral valve annulus. The white line depicting the distance measured between coronary sinus (CS) and mitral valve annulus. CxA, crcumflex artery.
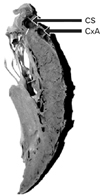
Fig. 2
Six longitudinal sections showing the sections of coronary sinus at point 1 to 4 and great cardiac vein (GCV) at point 5 and 6. The sections of the veins are marked by arrows. Arrowheads pointing at the sections of circumflex coronary artery. GCV is seen related superficially to the circumflex artery at point 6 and superior to it at point 5.

Fig. 3
Great cardiac vein (course marked by arrowheads) crossing the circumflex coronary artery (arrow) superficially.
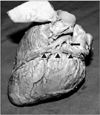
Fig. 4
Great cardiac vein (course marked by arrowheads) crossing left marginal artery (LMA) superficially.
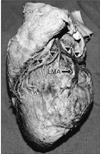
Fig. 5
Ventricular branches of circumflex coronary artery (white arrows) passing deep to the great cardiac vein (GCV) and coronary sinus (CS) to reach the left ventricle.
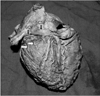
Table 1
The range, mean and standard deviation of the distance of coronary sinus/great cardiac vein from mitral valve annulus at point 1–6

| Point | Range (mm) | Mean±standard deviation (mm) |
|---|---|---|
| 1 | 8.04-10.56 | 9.39±0.52 |
| 2 | 9.78-11.68 | 10.78±0.52 |
| 3 | 8.36-10.98 | 9.64±0.48 |
| 4 | 8.28-10.90 | 9.27±0.57 |
| 5 | 6.03-10.56 | 7.21±1.08 |
| 6 | 5.27-10.90 | 6.39±1.30 |
References
1. Nicolini F, Agostinelli A, Vezzani A, Molardi A, Benassi F, Gallingani A, Romano G, Gherli T. Surgical treatment for functional ischemic mitral regurgitation: current options and future trends. Acta Biomed. 2015; 86:17–26.
2. Stolfo D, Merlo M, Pinamonti B, Poli S, Gigli M, Barbati G, Fabris E, Di Lenarda A, Sinagra G. Early improvement of functional mitral regurgitation in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2015; 115:1137–1143.
3. Ciarka A, Van de Veire N. Secondary mitral regurgitation: pathophysiology, diagnosis, and treatment. Heart. 2011; 97:1012–1023.
4. Calafiore AM, Iacò AL, Gallina S, Al-Amri H, Penco M, Di Mauro M. Surgical treatment of functional mitral regurgitation. Int J Cardiol. 2013; 166:559–571.
5. Jensen H. Surgical treatment of functional ischemic mitral regurgitation. Dan Med J. 2015; 62:B4993.
6. Fino C, Iacovoni A, Ferrero P, Merlo M, Bellavia D, D'Elia E, Miceli A, Senni M, Caputo M, Ferrazzi P, Galletti L, Magne J. Determinants of functional capacity after mitral valve annuloplasty or replacement for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015; 149:1595–1603.
7. Maniu CV, Patel JB, Reuter DG, Meyer DM, Edwards WD, Rihal CS, Redfield MM. Acute and chronic reduction of functional mitral regurgitation in experimental heart failure by percutaneous mitral annuloplasty. J Am Coll Cardiol. 2004; 44:1652–1661.
8. Liddicoat JR, Mac Neill BD, Gillinov AM, Cohn WE, Chin CH, Prado AD, Pandian NG, Oesterle SN. Percutaneous mitral valve repair: a feasibility study in an ovine model of acute ischemic mitral regurgitation. Catheter Cardiovasc Interv. 2003; 60:410–416.
9. Kaye DM, Byrne M, Alferness C, Power J. Feasibility and shortterm efficacy of percutaneous mitral annular reduction for the therapy of heart failure-induced mitral regurgitation. Circulation. 2003; 108:1795–1797.
10. Siminiak T, Wu JC, Haude M, Hoppe UC, Sadowski J, Lipiecki J, Fajadet J, Shah AM, Feldman T, Kaye DM, Goldberg SL, Levy WC, Solomon SD, Reuter DG. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail. 2012; 14:931–938.
11. Sahni D, Randhawa A, Aggarwal A, Rohit MK. Spatial relationship of coronary sinus-great cardiac vein with adjoining anatomic structures: a key element in predicting the success of percutaneous transvenous mitral annuloplasty. J Heart Valve Dis. 2014; 23:184–192.
12. Maselli D, Guarracino F, Chiaramonti F, Mangia F, Borelli G, Minzioni G. Percutaneous mitral annuloplasty: an anatomic study of human coronary sinus and its relation with mitral valve annulus and coronary arteries. Circulation. 2006; 114:377–380.
13. Labriola C, Greco F, Braccio M, Dambruoso PP, Labriola G, Paparella D. Percutaneous coronary sinus catheterization with the ProPlege catheter under transesophageal echocardiography and pressure guidance. J Cardiothorac Vasc Anesth. 2015; 29:598–604.
14. Menardi E, Ballari GP, Goletto C, Rossetti G, Vado A. Characterization of ventricular activation pattern and acute hemodynamics during multipoint left ventricular pacing. Heart Rhythm. 2015; 12:1762–1769.
15. El-Maasarany S, Ferrett CG, Firth A, Sheppard M, Henein MY. The coronary sinus conduit function: anatomical study (relationship to adjacent structures). Europace. 2005; 7:475–481.
16. Yamanouchi Y, Igawa O, Hisatome I. Activation mapping from the coronary sinus may be limited by anatomic variations. Pacing Clin Electrophysiol. 1998; 21(11 Pt 2):2522–2526.
17. Lansac E, Di Centa I, Al Attar N, Messika-Zeitoun D, Raffoul R, Vahanian A, Nataf P. Percutaneous mitral annuloplasty through the coronary sinus: an anatomic point of view. J Thorac Cardiovasc Surg. 2008; 135:376–381.
18. Harnek J, Webb JG, Kuck KH, Tschope C, Vahanian A, Buller CE, James SK, Tiefenbacher CP, Stone GW. Transcatheter implantation of the MONARC coronary sinus device for mitral regurgitation: 1-year results from the EVOLUTION phase I study (Clinical Evaluation of the Edwards Lifesciences Percutaneous Mitral Annuloplasty System for the Treatment of Mitral Regurgitation). JACC Cardiovasc Interv. 2011; 4:115–122.
19. Sack S, Kahlert P, Bilodeau L, Pièrard LA, Lancellotti P, Legrand V, Bartunek J, Vanderheyden M, Hoffmann R, Schauerte P, Shiota T, Marks DS, Erbel R, Ellis SG. Percutaneous transvenous mitral annuloplasty: initial human experience with a novel coronary sinus implant device. Circ Cardiovasc Interv. 2009; 2:277–284.
20. Machaalany J, Bilodeau L, Hoffmann R, Sack S, Sievert H, Kautzner J, Hehrlein C, Serruys P, Sénéchal M, Douglas P, Bertrand OF. Treatment of functional mitral valve regurgitation with the permanent percutaneous transvenous mitral annuloplasty system: results of the multicenter international percutaneous transvenous mitral annuloplasty system to reduce mitral valve regurgitation in patients with heart failure trial. Am Heart J. 2013; 165:761–769.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download