Abstract
Understanding of morphological structures such as the sphenoid spine and pterygoid processes is important during lateral transzygomatic infratemporal fossa approach. In addition, osseous variations such as pterygospinous and pterygoalar bridges are significant in clinical practice because they can produce various neurological disturbances or block the passage of a needle into the trigeminal ganglion through the foramen ovale. Two hundred and eighty-four sides of Korean adult dry skulls were observed to carry out morphometric analysis of the lateral plate of the pterygoid process, to investigate, for the first time among Koreans, the incidence of the pterygospinous and pterygoalar bony bridges, to compare the results with those available for other regional populations, and to discuss their clinical relevance as described on literatures. The mean of maximum widths of the left and right lateral plates of the pterygoid process were 15.99 mm and 16.27 mm, respectively. Also, the mean of maximum heights of the left and right lateral plates were 31.02 mm and 31.01 mm, respectively. The ossified pterygospinous ligament was observed in 51 sides of the skulls (28.0%). Ossification of the pterygospinous ligament was complete in four sides (1.4%). In 47 sides (16.6%), the pterygospinous bridge was incomplete. The ossified pterygoalar ligament was observed in 24 sides of the skulls (8.4%). Ossification was complete in eight sides (2.8%) and incomplete in 16 sides (5.6%). This detailed analysis of the lateral plate of the pterygoid process and related ossification of ligaments can improve the understanding of complex clinical neuralgias associated with this region.
The sphenoid bone lies at the base of the skull between the frontal, temporal and occipital bones. It has a central body, paired greater and lesser wings that spread laterally from the body, and two pterygoid processes that extend vertically downward from the junction of the root of greater wing and body of sphenoid. Each pterygoid process comprises of a lateral and a medial pterygoid plate. The lateral pterygoid plate is broad, thin, and everted. Its lateral surface forms part of the medial wall of the infratemporal fossa and gives origin to the lower part of the lateral pterygoid muscle [1].
The infratemporal fossa is a surgically significant region. The lateral transzygomatic infratemporal fossa approach is commonly used by surgeons to explore the median external skull base and the superior part of the para- and retro-pharyngeal space [234]. This approach is occasionally complicated by morphological structures such as the sphenoid spine and pterygoid processes. In the case of wide lateral pterygoid plates, it is possible that they could cause embracement of neurovascular structures and difficulties for the administration of mandibular anesthesia [5].
Foramen ovale is an oval shaped opening, placed obliquely in the base of the skull. It is situated in the greater wing of sphenoid bone, close to the upper end of posterior margin of lateral pterygoid plate, medial to foramen spinosum and lateral to the foramen lacerum [1]. It connects the middle cranial fossa to the infratemporal fossa and transmits the mandibular nerve, accessory meningeal artery, lesser petrosal nerve, and emissary vein. Understanding the accurate location and dimensions of foramen ovale plays a vital role during certain diagnostic and surgical procedures like electroencephalographic analysis, microvascular decompression by percutaneous trigeminal rhizotomy, and percutaneous biopsy of cavernous sinus tumors [67].
The ossified ligaments of the inferior surface of the sphenoid bone which exist close to the foramen ovale have been described in several studies [89]. The pterygospinous ligament (Civinini's ligament) is formed by the pterygoid fascia which runs between the posterior margin of the lateral plate of the pterygoid process and the angular spine of the undersurface of the greater wing of the sphenoid bone. Ossification of the pterygospinous ligament forms a bony bridge (Civinini's bar) that connects the spine of the sphenoid with the inferior surface of the greater wing, to create the pterygospinous foramen (Civinini's foramen) [8]. The ossification of the pterygospinous ligament may entrap the neurovascular structures and this may be an obstacle for the mandibular nerve block [10].
The pterygoalar ligament (Hyrtl-Calori's ligament) is a thin bundle of dense connective tissue extending from the root of the lateral plate of pterygoid process to the infratemporal surface of the greater wing [11]. Ossification of the pterygoalar ligament results in the appearance of a bony bridge, called the pterygoalar bar, which occasionally forms a pterygoalar foramen (porus crotaphitico-buccinatorius) [12]. The existence of the pterygoalar bar has great clinical importance, as it can be responsible for the compression of the branches of the mandibular division of the trigeminal nerve [13].
These osseous variations may be important in clinical practice, either by producing various neurological disturbances [14], or by blocking the passage of a needle through the foramen ovale [8]. Transovale and intracisternal injections for trigeminal neuralgia may not be successful, due to an osseous bar that can hinder the horizontal transzygomatic method [15].
Therefore, the aims of this study were (1) to carry out morphometric analysis of the lateral plate of the pterygoid process, (2) to investigate, for the first time among Koreans, the incidence of the pterygospinous and pterygoalar bony bridges, (3) to compare the results with those available for other regional populations, and (4) to discuss their clinical relevance as described on literatures.
One hundred and forty-two Korean adult dry skulls of unspecified sex and age were obtained from the Department of Oral Anatomy, School of Dentistry, Pusan National University and Department of Anatomy, College of Medicine, The Catholic University of Korea. Skulls that showed evidence of obvious trauma or pathological conditions were excluded. Two hundred and eighty-four sides were observed for the incidence of both pterygospinous and pterygoalar bars and foramina. The gross appearance of the structures and their relations to foramen ovale were observed and photographs were taken. The criteria used to evaluate the existence of the pterygospinous and pterygoalar bridges was that of Hauser and De Stefano [16] and Peker et al. [17]. By modifying their methods, the classification was made, according to the degree of ossification of the ligaments between the bones, as follows (Fig. 1):
(1) Incomplete: elongation of the tubercles or spines, on one or both ones of the ligamentous attachments.
(2) Complete: fusion between the elongated tubercles or spines, thus yielding a foramen.
For the morphometric analysis of the lateral plate of the pterygoid process and nearby structures, five items were measured using 0.01 mm sensitive digital caliper (model CD-15CP, Mitutoyo, Kawasaki, Japan). Descriptions for all 5 measurements of the skull are given in Fig. 2 and Table 1. All measurements were made by the same person and repeated three times, and the mean values of the three measurements were taken.
Left and right asymmetry of the categorical variables was investigated using the unpaired Student's t test and their correlation was examined using Pearson's correlation. Statistical analysis was performed using SPSS version 15.0 (PASW Statistics 18, SPSS Inc., Chicago, IL, USA) and the level of significance was set at P<0.05.
The results of all measurements on the lateral plate of the pterygoid process and its neighboring structures are shown in Table 2. The mean of maximum widths of the left and right lateral plates of the pterygoid process were 15.99 mm and 16.27 mm, respectively. The mean of maximum heights of the left and right lateral plates were 31.02 mm and 31.01 mm, respectively. There were four sides of the skulls (1.4%) that had enlarged lateral plates with a width over 25 mm (range, 25.94 to 30.23 mm), two unilateral and one bilateral. The mean distance between the spine of the sphenoid bone and the posterior margin of the lateral pterygoid plate was 10.22 mm on left side and 10.79 mm on right side. The mean distance from the center of foramen ovale to the orifice of the vomerorostral canal on midline of the skull was 27.20 mm on left and 26.66 mm on right, and the mean distance from center of the foramen ovale to the root of zygomatic arch was 33.34 mm on left and 33.88 mm on right.
Through Student's t test for the comparison of the right and left, statistically significant differences in two items, d and e (Table 2) were observed. The distance from the center of foramen ovale to the orifice of the vomerorostral canal (d) on the left was longer than that of the right, and the distance from the center of foramen ovale to the root of zygomatic arch (e) on the right was longer than that of the left. In addition, Pearson's correlation analysis was applied to find out the correlation between the items measured, and the ones that have statistical significance (P<0.05) are summarized in Table 3.
The ossified pterygospinous ligament was observed in 51 sides of the skulls (18.0%). The ossification was complete in four sides (1.4%), two unilaterally and one bilaterally (Fig. 3B). In 47 sides of the skulls (16.6%), the pterygospinous bridge was incomplete, 23 unilaterally and 12 bilaterally (Fig. 3A). There was no statistically significant difference between the existence of the ossified pterygospinous ligament and the sides of the skulls (P=0.792). The data are summarized in Tables 4 and 5.
The ossified pterygoalar ligament was observed in 24 sides of the skulls (8.4%). The ossification was complete in eight sides (2.8%), and all of them were unilateral (Fig. 4B). In 16 sides (5.6%), the pterygoalar bridge was incomplete, 10 unilateral and 3 bilateral (Fig. 4A). There was no statistically significant difference between the existence of the ossified pterygoalar ligament and the sides of the skulls (P=0.647). The data are summarized in Tables 4 and 5.
The lateral plate of the pterygoid process forms the medial wall of the infratemporal fossa. Elongation of the lateral plate of the pterygoid process could result in weakening of the medial pterygoid muscle and paresthesia of the inner aspect of the cheek [18]. Also, compression of the lingual nerve by the elongated lateral plate could lead to a weakening of taste transmission from the taste buds located on the anterior two thirds of the tongue [19]. In case of extremely large lateral plate, the lingual and inferior alveolar nerves in the region of the infratemporal fossa are forced to take a long curved course, following the shape of the enlarged plate. During contraction of the pterygoid muscles, both nerves can be compressed [18]. Krmpotic-Nemanic et al. [19] observed that the appearance of the large lateral plate was in general unilateral. In three out of 142 examined dry skulls, lateral plates of the pterygoid process were measured to be over 25 mm and considered enlarged for this study. The anatomical study of Skrzat et al. [20] proposed that the elongation of the lateral pterygoid plate could result in weakening of the medial pterygoid muscle and paresthesia of the buccal region. The lateral plate of the pterygoid process forms an important landmark for mandibular anesthesia and any anomaly is bound to confuse anesthetists [18]. Therefore, a wider lateral pterygoid plate may pose difficulty for surgeons exploring the para- and retro-pharyngeal space [21].
The sphenoid bone presents a series of intrinsic ligaments such as interclinoid, caroticoclinoid, pterygospinous, and pterygoalar ligaments [22]. Italian anatomist F. Civinini (1805–1844) first described and named the term pterygospinous ligament or ligament of Civinini. The ossified ligament projects as a bar medially, laterally or sometimes across the foramen ovale and may interfere with percutaneous injections into the trigeminal ganglion [10]. The pterygospinous bridge passes medially to the foramen spinosum and crosses the foramen ovale at an angle of 20° to 40° to the sagittal plane [23]. Rouviere and Delmas [24] stated that the pterygospinous ligament divides the sphenomandibular ligament into two independent parts, of which the interpterygoid fascia is thin. Lateral to the interpterygoid fascia is another fibrous sheet inserted in the greater wing of the sphenoid bone and the superior segment of the posterior edge of the lateral plate of the pterygoid process. Its superior edge becomes flat and forms an innominate ligament as described by Hyrtl in 1862 [25], and posteriorly called pterygoalar ligament which when it is ossified, molds the pterygoalar foramen [13]. The ossification of these ligaments with different clinical implications has been reported variously. The present study analyzed the presence of complete or incomplete ossification of the pterygospinous and pterygoalar ligaments. These formations occupy a deep and high portion in the infratemporal fossa, establishing important relationships with the mandibular nerve and its branches, the otic ganglion, the middle meningeal artery and vein, the tympanic nerve, and the medial and lateral pterygoid muscles [22]. These are compressed against the bone formations and are capable of generating clinically important alterations [826]. Pterygospinous ligaments are fibrous bands formed by thickened cranial part of fascia located between the lateral and medial pterygoid muscles.
There are several reports in the literature that indicate the frequency and types of the pterygospinous and pterygoalar bridges in different populations, and the results from the comparison among populations are summarized in Table 6. Lepp and Sandner [27] reported that these ossified ligaments were present in about 8%–10% of the population and that the pterygoalar ligament was ossified more often than the pterygospinous ligament. In this study, the complete pterygoalar bridge (2.8%) was observed more often than the pterygospinous bridge (1.4%), whereas the incomplete pterygospinous bridge (16.6%) was seen more frequently than the pterygoalar bridge (5.6%). Kapur et al. [5] observed complete pterygospinous bridges in 1.2% of the examined Croatian skulls and incomplete ones in 5.4%, while Peker et al. [17] in an Anatolian population observed 5.6% and 12.3%, respectively. Moreover, Antonopoulou et al. [13] observed 2% and 25%, respectively, in Greek population. In this study of a Korean population, complete pterygospinous bridges were found in 1.4% and incomplete ones in 16.6%. This result seems to be lower than the figures obtained in Antonopoulou et al.'s study [13] of the Greek population and similar to the results of Croatian population of Kapur et al. [5] and Anatolians of Peker et al. [17]. For pterygoalar bridges, Peker et al. [17] observed complete ones in 4.9% and incomplete ones in 5.1% of the Anatolian skulls. In contrast, Kapur et al. [5] in a Croatian population and Pinar et al. [28] in Turkish reported a lower frequency for both the complete (2.4% and 1.1%) and incomplete pterygoalar bridges (4.4% and 4.98%), respectively. In this study, complete pterygoalar bridges were found in 2.8% of the skulls and incomplete bridges in 5.6%. Overall, data from several studies show that the incidence of complete and incomplete pterygoalar bridge is variable and lies among the range between 0.98% to 5.5% and 3.6% to 8%, respectively. Within this range, the percentages for both the complete and incomplete pterygoalar bridges of Koreans were in the average (Table 6).
The presence of these ossified formations at the lateral surface of the lateral pterygoid plate may impede access to the foramen ovale during induction of anesthesia of the trigeminal nerve [5]. The pterygoalar ligament can potentially press on the deep temporal, lateral pterygoid and buccal nerves (branches of the anterior trunk of the mandibular division of the trigeminal nerve), and on branches of the auriculotemporal nerve [19]. Such compression may cause chewing disorders, pain, numbness of the buccal region and changes to the parotid gland salivation [17].
In conclusion, a detailed analysis of the lateral plate of the pterygoid process and related ossification of ligaments inserted can improve our understanding of the complex clinical neuralgias affecting this region. This study was done in the hopes for improving the effectiveness of surgical and anesthetic procedures.
Figures and Tables
Fig. 1
Classification of the pterygospinous and pterygoalar bridges including degree of completeness. (A) Incomplete pterygospinous bridging (arrows). (B) Complete pterygospinous bridging. (C) Incomplete pterygoalar bridging. (D) Complete pterygoalar bridging. AT, articular tubercle; FO, foramen ovale; FS, foramen spinosum; LPP, lateral plate of the pterygoid process; MF, mandibular fossa; SP, styloid process.
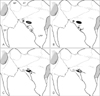
Fig. 2
(A, B) Illustrations showing the morphometric items measured on the lateral plate of the pterygoid process and around structures. Written descriptions of these measurements can be found in Table 1.
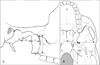
Fig. 3
Ossified pterygospinous ligament. Incomplete pterygospinous bridges (arrows) (A) and pterygospinous foramina (arrowheads) (B) formed by complete pterygospinous bridges. FO, foramen ovale; FS, foramen spinosum; LPP, lateral plate of the pterygoid process.

Fig. 4
Ossified pterygoalar ligament. Incomplete pterygoalar bridge (arrows) (A) and pterygoalar foramen (arrow head) (B) formed by complete pterygoalar bridge. FO, foramen ovale; LPP, lateral plate of the pterygoid process.

Table 1
Description of each skull measurements

Table 2
Measurements of the lateral plate of the pterygoid process and around structures

Table 3
Correlation between the items measured

Table 4
Distribution of the pterygospinous and pterygoalar bridges related to the appearance of osseous variations

| Pterygospinous bridge | Pterygoalar bridge | |
|---|---|---|
| Absence | 233 (82.0) | 260 (91.6) |
| Incomplete | 47 (16.6) | 16 (5.6) |
| Complete | 4 (1.4) | 8 (2.8) |
| Total | 284 (100) | 284 (100) |
Table 5
Distribution of the pterygospinous and pterygoalar bridges according to the sides (right and left) of skulls

Table 6
Incidence of pterygospinous and pterygoalar bridges according to the authors

| Type of ossification | Pterygospinous (%) | Pterygoalar (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete | Incomplete | Complete | Incomplete | |||||||
| Author | Year | Population/Specimen | Left | Right | Left | Right | Left | Right | Left | Right |
| Chouke [11] | 1949 | European/6,000 DS | - | - | - | - | 7.72 | 14.79 | ||
| Chouke and Hodes [15] | 1951 | American/1,234 Pts | - | - | - | - | 2.9 | 2.35 | - | - |
| Priman and Etter [29] | 1959 | American/250 DS | 3 | - | - | 2.5 | ||||
| Tebo [25] | 1968 | Indian/516 DS | 3.9 | 33 | - | - | - | - | ||
| Shaw [14] | 1993 | 454 DS | 16.1 | - | - | - | - | - | - | |
| Krmpotic-Nemanic et al. [19] | 1999 | Croatian/100 DS | 20.1 | - | - | - | - | - | - | |
| Kapur et al. [5] | 2000 | Croatian/305 DS | 0.98 | 0 | 6.22 | 4.59 | 3.90 | 0.98 | 5.24 | 3.6 |
| Peker et al. [17] | 2002 | Anatolian/452 DS | 6.4 | 4.7 | 12.6 | 11.9 | 5.5 | 4.2 | 4.9 | 5.3 |
| Pinar et al. [28] | 2004 | Turkish/361 DS | 3.32 | 1.31 | 9.7 | 0 | 1.10 | 0 | 4.98 | 0 |
| von Ludinghausen et al. [26] | 2006 | Japanese/100 DS | 20.4 (6% complete pterygospinous bar) | - | - | - | - | |||
| Das and Paul [30] | 2007 | Indian/50 DS | - | - | - | 2 | - | - | - | - |
| Nayak et al. [31] | 2007 | Indian/416 DS | 2.88 | 2.88 | 1.92 | 0 | - | - | - | - |
| Antonopoulou et al. [13] | 2008 | Greek/50 DS | 2 | 2 | 28 | 22 | 2 | 0 | 8 | 6 |
| This study | 2014 | Korean/142 DS | 2.1 | 0.7 | 18.3 | 14.8 | 1.4 | 4.2 | 7.7 | 3.5 |
Acknowledgements
We would like to express our thanks to Dr. Kwan-Hyun Youn for the schematic illustrations and Ms. YunJeong Choi for reviewing this manuscript. This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111442).
References
1. Standring S, Borley NR, Collins P, Crossman AR, Gatzoulis MA, Healy JC, Johnson D, Mahadevan V, Newell RL, Wigley K. Gray's anatomy: the anatomical basis of clinical practice. 40th ed. London: Churchill Livingstone;2008. p. 415.
2. Donovan MG, Ondra SL, Illig JJ, Dickerson NC. Combined transmandibular-zygomatic approach and infratemporal craniotomy for intracranial skull base tumors. J Oral Maxillofac Surg. 1993; 51:754–758.
3. Mickey B, Close L, Schaefer S, Samson D. A combined frontotemporal and lateral infratemporal fossa approach to the skull base. J Neurosurg. 1988; 68:678–683.
4. Prades JM, Timoshenko A, Merzougui N, Martin C. A cadaveric study of a combined trans-mandibular and trans-zygomatic approach to the infratemporal fossa. Surg Radiol Anat. 2003; 25:180–187.
5. Kapur E, Dilberović F, Redzepagić S, Berhamović E. Variation in the lateral plate of the pterygoid process and the lateral subzygomatic approach to the mandibular nerve. Med Arh. 2000; 54:133–137.
6. Gerber AM. Improved visualization of the foramen ovale for percutaneous approaches to the gasserian ganglion. Technical note. J Neurosurg. 1994; 80:156–159.
7. Gusmao S, Oliveira M, Tazinaffo U, Honey CR. Percutaneous trigeminal nerve radiofrequency rhizotomy guided by computerized tomography fluoroscopy. Technical note. J Neurosurg. 2003; 99:785–786.
8. Peuker ET, Fischer G, Filler TJ. Entrapment of the lingual nerve due to an ossified pterygospinous ligament. Clin Anat. 2001; 14:282–284.
9. Srisopark SS. Ossification of some normal ligaments of the human skull which produce new structures: the pterygospinous and pterygoalar bars and foramina, and the caroticoclinoid foramen. J Dent Assoc Thai. 1974; 24:213–224.
10. Saran RS, Ananthi KS, Subramaniam A, Balaji MT, Vinaitha D, Vaithianathan G. Foramen of civinini: a new anatomical guide for maxillofacial surgeons. J Clin Diagn Res. 2013; 7:1271–1275.
11. Chouke KS. Injection of mandibular nerve and gasserian ganglion: an anatomic study. Am J Surg. 1949; 78:80–85.
12. Patnaik VV, Singla RK, Sanju B. Bilateral pterygo-alar bar and porus crotaphitico buccinatorius: a case report. J Anat Soc India. 2001; 50:161–162.
13. Antonopoulou M, Piagou M, Anagnostopoulou S. An anatomical study of the pterygospinous and pterygoalar bars and foramina: their clinical relevance. J Craniomaxillofac Surg. 2008; 36:104–108.
14. Shaw JP. Pterygospinous and pterygoalar foramina: a role in the etiology of trigeminal neuralgia? Clin Anat. 1993; 6:173–178.
15. Chouke KS, Hodes PJ. The ptergoalar bar and its recognition by roentgen methods in trigeminal neuralgia. Am J Roentgenol Radium Ther. 1951; 65:180–182.
16. Hauser G, De Stefano GF. Epigenetic variants of the human skull. Stuttgart: E. Schweizerbart'sche Verlagsbuchhandlung. 1989. p. 156–161.
17. Peker T, Karaköse M, Anil A, Turgut HB, Gülekon N. The incidence of basal sphenoid bony bridges in dried crania and cadavers: their anthropological and clinical relevance. Eur J Morphol. 2002; 40:171–180.
18. Piagkou MN, Demesticha T, Piagkos G, Androutsos G, Skandalakis P. Mandibular nerve entrapment in the infratemporal fossa. Surg Radiol Anat. 2011; 33:291–299.
19. Krmpotić-Nemanić J, Vinter I, Hat J, Jalsovec D. Mandibular neuralgia due to anatomical variations. Eur Arch Otorhinolaryngol. 1999; 256:205–208.
20. Skrzat J, Walocha J, Srodek R. An anatomical study of the pterygoalar bar and the pterygoalar foramen. Folia Morphol (Warsz). 2005; 64:92–96.
21. Lang J. Skull base and related structures: atlas of clinical anatomy. Stuttgart: Schattauer;1995. p. 55–57.
22. Suazo GI, Zavando MD, Smith RL. Anatomical study of the pterygospinous and pterygoalar bony bridges and foramens in dried crania and its clinical relevance. Int J Morphol. 2010; 28:405–408.
23. Newton TH, Potts DG. Radiology of the skull and brain. St. Louis, MO: Mosby;1971. p. 307.
24. Rouviere H, Delmas A. Descriptive, topographical, and functional human anatomy. Barcelona: Masson;1999.
25. Tebo HG. The pterygospinous bar in panoramic roentgenography. Oral Surg Oral Med Oral Pathol. 1968; 26:654–657.
26. von Ludinghausen M, Kageyama I, Miura M, Alkhatib M. Morphological peculiarities of the deep infratemporal fossa in advanced age. Surg Radiol Anat. 2006; 28:284–292.
27. Lepp FH, Sandner O. Anatomic-radiographic study of ossified pterygospinous and "innominate" ligaments. Oral Surg Oral Med Oral Pathol. 1968; 26:244–260.
28. Pinar Y, Arsu G, Aktan Ikiz ZA, Bilge O. Pterygospinous and pterygoalar bridges. Sendrom. 2004; 16:66–69.
29. Priman J, Etter LE. The pterygospinous and pterygoalar bars. Med Radiogr Photogr. 1959; 35:2–6.
30. Das S, Paul S. Ossified pterygospinous ligament and its clinical implications. Bratisl Lek Listy. 2007; 108:141–143.
31. Nayak SR, Saralaya V, Prabhu LV, Pai MM, Vadgaonkar R, D'Costa S. Pterygospinous bar and foramina in Indian skulls: incidence and phylogenetic significance. Surg Radiol Anat. 2007; 29:5–7.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download