Abstract
Pax6, a paired homeobox DNA binding protein, has been found to be expressed in the cerebellum in both granule cells and their precursors in the external granular layer (EGL). In this study we have traced Pax6 expression through embryonic development in mice by using a polyclonal antibody against Pax6 and used it to study the cellular dispersal pattern of the EGL. During dispersal the EGL was thicker and Pax6 expression was more intense on the rostral side of the lateral corners of the cerebellum. Pax6 immunoreactive cells were found to be migrating from the EGL during the early stage of EGL dispersal, which suggested the early inward migration of granule cells. Double staining with various markers confirmed that the early-migrating cells are not Purkinje cells, interneurons or glia. Although the Pax6 immunoreactive cells within the cerebellum were not apparently proliferating, NeuN, a marker for postmitotic granule cells, was not expressed in these cells until E16. Furthermore, granule cells were observed migrating inwards from the EGL both during and after EGL dispersal. These early migrating granule cells populated the whole cerebellum. These findings offer novel views on specific stages of granule cell dispersal and migration.
The granule cells (GCs) of the cerebellum are the most numerous neurons of the entire central nervous system and lie within the internal granular layer (IGL), which is located below a monolayer of Purkinje cells (PCs). Above the PC layer lies the molecular layer, where the GCs form parallel fibers that make excitatory synapses on PC dendrites. GC precursors disperse from the upper rhombic lip (URL) in a rostral direction over the dorsal surface of the cerebellum to form the external granular layer (EGL). Altman and Bayer have extensively studied the development of the EGL in rats and reconstructed the EGL dispersal pathway over the cerebellum (Altman, 1972; Altman & Bayer, 1985; 1997). They found that the EGL proliferates from the rhombic lip at E17 in an anterodorsal direction. Medial (or vermal) EGL cells disperse rostrally and lateral (or hemispheric) cells disperse medially.
A paired homeobox DNA binding protein, Pax6, is expressed in GCs and their precursors. A mutation in the Pax6 gene was found in aniridia patients (Hanson et al., 1993). These patients do not develop irises. Mice and rats with a null mutation in Pax6 were identified and dubbed Small Eye. Animals heterozygous for the mutation have smaller eyes than their normal littermates. Homozygous animals do not develop eyes or nasal cavities, and they die shortly after birth (Hill et al., 1991). It was found that Pax6 was crucial for eye development (Hanson & Van Heyningen, 1995). Pax6 has also been found to play a major role in the development of pancreatic islets (Beimesche et al., 1999). In the central nervous system, Pax6 has been implicated in axon pathfinding, neuron migration and the formation of axon tracts and projections (Mastick et al., 1997; Kawano et al., 1999). In the cerebellum, Pax6 is expressed in both the EGL and the IGL (Engelkamp et al., 1999; Yamasaki et al., 2001). Cerebella of homozygous Small Eye mice have a narrower vermis and lack foliation when compared with wild-type littermates. Though dispersal of the EGL appeared normal in Small Eye, GCs failed to migrate transversely and completely failed to migrate inwards (Engelkamp et al., 1999). Yamasaki et al. found that this is due to a cell autonomous defect in polarization in mutant GCs. Because of this defect, mutant GCs do not develop a leading process and migrate from the EGL in random directions (Yamasaki et al., 2001).
Research has been done on the migratory pathways of GCs for over a century. However, previous methods, involving either cresyl violet (Nissl) staining or 3H-thymidine birthdating, were not conclusive (e.g. Altman & Bayer, 1997; Beierbach, 2001). When specific antibodies were used, either only postnatal tissue was labeled (Karam et al., 2001), or, when embryonic tissue was labeled, the studies focused on Pax6 expression in the whole brain was studied or focused on aspects of cerebellar development separate from EGL dispersal (Engelkamp et al., 1999; Jensen et al., 2004). Therefore, the aim of this study was to investigate the dispersal pattern of the EGL in more detail. By labeling during early embryonic development with an antibody against Pax6, we found that Pax6-immunoreactive neurons in the developing cerebellum are indeed early inward-migrating granule cells.
Pregnant CD1 mice were purchased from Charles River Laboratories (Montréal, Canada). The breeding date was defined as E0. Mice were housed according to local governmental and institutional animal care guidelines and fed ad libitum. Dams were sacrificed by cervical dislocation at pregnancy dates E11 to E18, and the embryos were removed. The embryonic brains were dissected, immersion fixed in 4% paraformaldehyde (PFA) (EMD Biosciences, San Diego, USA), 0.9% NaCl and 0.1 M phosphate buffer (pH 7.4) (PBS) (Sigma, St. Louis, USA), and stored at 4℃ until further processing.
The following primary antibodies were used:
- rabbit anti-Pax6 (used diluted 1:2000, Chemicon Inc. Temecula USA), which recognizes granule cells (Engelkamp et al., 1999; Yamasaki et al., 2001)
- mouse anti-NeuN (1 : 2,000, Chemicon Inc.), which recognizes differentiated granule cells (Weyer & Schilling, 2003)
- rabbit anti-Pax2 (1 : 1,000, Chemicon Inc.), which recognizes stellate and basket cell precursors (Maricich & Herrup, 1999)
Embryonic cerebellums were obtained from pregnant mice. Embryonic cerebellums were quickly washed with 0.9% NaCl in 0.1 M phosphate buffer (PBS: pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 48 hours. The cerebella were then cryoprotected through a series of buffered sucrose solutions: 10% (2 hrs), 20% (2 hrs) and 30% (overnight). Series of 40 µm thick transverse sections were cut through the extent of the cerebellum on a cryostat and collected for free-floating immunohistochemistry. Briefly, tissue sections were washed thoroughly, blocked with 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA) and then incubated in 0.1 M phosphate buffered saline (PBS) containing 0.1% Triton-X and the primary antibody for 16~18 hours at 4℃. Secondary incubation in horseradish peroxidase (HRP) conjugated goat anti-rabbit or HRP conjugated goat anti-mouse antibodies (all diluted 1 : 200 in PBS; Jackson ImmunoResearch Laboratories, West Grove, PA) was subsequently performed for 2 hours at room temperature. Diaminobenzidine (DAB, 0.5 mg/ml) was used to visualize the reaction product. Finally, sections were dehydrated through an alcohol series, cleared in xylene and coverslipped with Entellan mounting medium (BDH Chemicals, Toronto, ON, Canada).
Cerebellar sections were also processed for fluorescent immunohistochemistry. Tissue sections were washed, blocked in PBS containing 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA), then incubated in both primary antibodies overnight at room temperature, rinsed, and incubated for 2 hours at room temperature in a mixture of Alexa 546-conjugated goat anti-rabbit Ig and Alexa 488-conjugated goat anti-mouse Ig (Molecular Probes Inc., Eugene, OR), all diluted 1 : 2,000. After several rinses in 0.1 M PBS, sections were coverslipped in non-fluorescing mounting medium (Fluorsave Reagent, Calbiochem, La Jolla, CA).
Dams were injected with 100 µl 18.2 mg/mL bromodeoxyuridine (BrdU, Sigma) on pregnancy day 15 and sacrificed after a survival period of 10 minutes according to the protocol of Miller and Nowakowski (1988). To label granule cells cells, dams pregnant for 10 days were injected with 100 µl 18.2 mg/ml BrdU and sacrificed on pregnancy day 15. The embryonic cerebella were removed, fixed, sectioned and stored as described above. Prior to immunohistochemistry, sections were treated with HCl (1 N, Titristar EMD Sciences) for 30 minutes at RT. Mouse anti-BrdU (1 : 5,000, G3G4, DSHB, Iowa, USA) was used to detect incorporated BrdU.
Photomicrographs were captured with a SPOT cooled color digital camera (Diagnostic Instruments Inc.) mounted on a Zeiss microscope and assembled in Adobe Photoshop (version 9). The images were cropped and corrected for brightness and contrast but not otherwise manipulated.
Cerebella at embryonic age E11 to E18 were sectioned horizontally or sagittally and stained for Pax6. No cells immunoreactive for Pax6 are observed in the cerebellum at E11 (Fig. 1A~C). At E12, a rostrodorsal group of cells immunoreactive for Pax6 is seen in both lateral and paramedial sections (Fig. 1E, F). At E12, a single layer of Pax6-immunoreactive cells on the dorsal surface of the cerebellum is observed that connects the rostral group of Pax6-immunoreactive cells to the upper rhombic lip (URL) (Fig. 1D~F).
The dorsal layer of Pax6-immunoreactive cells increases to 3 cells thick by E13 (Fig. 2B) and to 6 cells thick by E14 (Fig. 2C), and is now referred to as the EGL. In medial sections, no dorsal layer of Pax6-immunoreactive cells is observed until E14 (Fig. 2D), suggesting that paramedial and lateral EGL dispersal may start earlier than medial EGL dispersal. In lateral and paramedial sections, Pax6-positive cells within the cerebellum are located more rostrally than the leading edge of the EGL (Fig. 4E, F).
At E15, the rostral groups of Pax6-positive cells are most distinguishable (Fig. 3A~C). At E16, the inward migrating rostral group of Pax6-immunoreactive cells is less well defined in paramedial sections (Fig. 3E). At E17, we observed an area in the center of the cerebellum in paramedial sections that contains no Pax6-positive cells (Fig. 4B). Interestingly, this is not the case in the lateral sections, where a group of Pax6-immunoreactive cells can still be distinguished at the rostral end of the cerebellum at E17 and E18 (Fig. 4C, F). This suggests that Pax6 expression is down-regulated in at least the medial nucleus.
The rostral Pax6-positive group of cells within the cerebellum is most distinguishable at E15. To determine the identity of these cells, dams were injected with a non-lethal dose of BrdU on E10, sacrificed on E15, and sections were immunofluorescently double labeled for Pax6 and BrdU. In paramedial sections, many, but not all, of the rostral Pax6-positive group of cells are also immunoreactive for BrdU (Fig. 5B, C). It general, it appeared that most double labeled cells are more ventrally located than cells immunoreactive for Pax6 alone. Two possible explanations are that the latter cells were born either before BrdU injection, or after, when no more BrdU was available. These data suggest that these cells were not actively dividing at the time of BrdU injection.
To determine the identity of the other Pax6-immunoreactive cells within the cerebellum that appeared to have migrated inwards from the EGL, E15 sagittal and horizontal sections were immunostained with several cell type-specific markers. When sections are stained for Pax2, immunoreactive somata are seen scattered from the rostral end of the VZ to the dorsocaudal part of the cerebellum (Fig. 5A), which corresponds with the known migration pattern of stellate- and basket cell precursors (Maricich & Herrup 1999).
When E15 sagittal sections are immunolabeled for NeuN, an antigenic marker of mature GCs (Weyer & Schilling, 2003), no immunoreactive profiles are seen in the cerebellum, suggesting that the Pax6-positive cells within the cerebellum are not comprised of mature GCs (data not shown). To investigate when the Pax6-positive cells within the cerebellum mature into GCs, E16, E17 and E18 sagittal sections were double immunolabeled for Pax6 and NeuN. NeuN expression is first observed at E16 in a group of cells extending from the center of the cerebellum to the dorsal surface of the posterior lobe. All cells that are immunoreactive for NeuN are also immunoreactive for Pax6 (Fig. 5D). At E17 and E18, the number of double-positive cells increases (Fig. 5E, F) but remain in the same location, i.e. in a cluster extending from the center of the cerebellum into future lobules VII and VIII.
In this study we have shown that Pax6 is expressed in both EGL cells and their derivatives, as well as in migrating deep cerebellar nuclei (DCN) cells. When Pax6 expression was studied more closely, details about the migrating DCN, the EGL and its dispersal were observed.
It is widely accepted that the inward migration of granule cells from the EGL does not begin until birth. Previous studies have shown that granule cells migrate primarily through raphes between postnatal days (P)0 and P7. After P7, no raphes are observed and granule cells migrate inwards en masse (Luckner et al., 2001; Karam et al., 2001). However, in this study we found that postmitotic Pax6-positive cells migrate from the EGL as early as E14, and double immunostaining with various cell markers suggested that these cells are likely GCs. These early inward migrating GCs did not appear to migrate in raphes but rather were scattered throughout the whole cerebellum. After E14, granule cells continued to migrate from the EGL. At E15, the GCs were found from the EGL to the ventral neuroepithelium, indicating that they had migrated past the location of the future IGL. From E16 to E18, Purkinje cells became more concentrated below the EGL, and the number of GCs seen in this layer decreased. At E18, GCs began to concentrate below the future PC layer in the future IGL, and no GCs were seen in the PC layer in most parts of the cerebellum.
Studies have sought to determine what signals GCs to migrate inward at specific locations. Cadherins and ephrins, two families of cell-adhesion molecules, are thought to play roles in determining the location of raphes. Raphes were found at the borders of PC clusters expressing different types of cadherins, Eph receptors and ephrin ligands (Karam et al., 2001; Luckner et al., 2001). In our study, GCs appeared to freely migrate inward until the PCs formed a layer. Therefore, we hypothesize that the inward migration of GCs is mechanically blocked by PCs between E16 and E18. In future studies, we plan to label P5 to P7 sections for Pax6 or RORα to investigate whether the start of the massive inward migration of GCs coincides with the transition from a multiple cell layer to a single layer of PCs.
Our finding that the early inward migrating cells from the EGL mature within the cerebellum led us to hypothesize that these cells may not be post-mitotic when they leave the EGL. Our BrdU data show that very few BrdU immunoreactive cells were observed At E15 (E15 injection and sacrificed 10 minutes later), and no Pax6 immunoreactive cells within the cerebellum were immunoreactive for BrdU. This suggests that Pax6-immunoreactive cells within the cerebellum were not proliferating at the time of BrdU exposure. Therefore we conclude that early inward migrating GCs, like the postnatally inward migrating GCs, are postmitotic, even though they do not express NeuN until E16. Taken together, our findings provide novel views on specific stages of granule cell dispersal and migration.
Figures and Tables
Fig. 1
Pax6 expression at embryonic day E11 and E12 in the cerebellum. The top row shows medial sections, the middle row shows paramedial sections and the bottom row shows lateral sections that correspond to mediolateral locations. Immunocytochemistry with an antibody against Pax6 was performed on sagittal sections. No Pax6 expression is observed at E11 (A~C). At E12 (D~F), cells immunoreactive for Pax6 are first seen in a single layer on the dorsal surface, as well as a small group of cells at the rostral end of the layer (arrow). Scale bar is 250 µm.
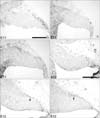
Fig. 2
Pax6 expression in E13 and E14 cerebella. At E13 (A~C), the dorsal layer has increased, and the rostral group has increased in size and number. At E14 (D~F), the EGL is first observed in the medial sections, and inward migration has begun. Ed-The authors should state what each row of images is referring to, even if it is the same as in Fig. 1. Also, the authors should state what the arrows are pointing to in panels (B, C, E, F) Scale bar is 250 µm.
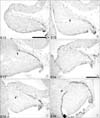
Fig. 3
Pax6 expression in E15 and E16 cerebella. At E15 (A~C), the rostral group of cells, as well as all other Pax6 immunoreactive cells within the cerebellum, has reached the ventral side of the cerebellum in the paramedial sections, and a discontinuity in EGL thickness is observed in the dorsocaudal corner of the cerebellum in the lateral sections (arrowheads). At E16 (D~F), this discontinuity is more obvious, and the rostral group of cells no longer expresses Pax6 and may have migrated caudally into the center of the cerebellum (asterisk). Ed-Similar to notes for Fig. 2, the authors should state what each row of images is referring to. Also, the authors should state what the arrows are pointing to. Scale bar is 250 µm.
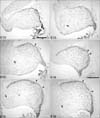
Fig. 4
Pax6 expression in E17 and E18 cerebella. At E17 (A~C) and E18 (D~F), a rostral group of Pax6 immunoreactive cells is only seen in lateral sections. From E15 to E18, the discontinuity remains in the same rostrocaudal location. Arrows point to the rostral group of Pax6 immunoreactive cells. Arrowheads point to the rostral and caudal end of the discontinuity in the EGL of lobule VIb and VII. Asterisks indicate the presumed location of the rostral group of cells that were immunoreactive for Pax6 before E16. Panels A and D show medial sections, B and E show paramedial sections, and C and F show lateral sections. Scale bar is 250 µm.
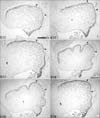
Fig. 5
Identification of the Pax6 immunoreactive cells within the cerebellum. Immunocytochemistry with an antibody against Pax2 shows a different migration pattern from Pax6 (A; rostral is left, dorsal is up). Double immunofluorescence labeling for BrdU (green) and Pax6 (red) of an E15 cerebellum that was injected with BrdU 10 minutes before sacrifice, shows all Pax6 immunoreactive cells within the cerebellum are postmitotic (B). Arrowheads in (B) point to cells that are immunoreactive for BrdU only. Double labeling with BrdU (green) and Pax6 (red) of an E15 cerebellum that was injected with BrdU at E10 shows that some cells of the rostral group of Pax6 immunoreactive cells are DCN cells (C). Arrowheads in (C) point to cells immunoreactive for both Pax6 and BrdU. Double immunofluorescence labeling for NeuN (green) and Pax6 (red) at E16 (D), E17 (E) and E18 (F) suggests that the Pax6 immunoreactive cells within the cerebellum are granule cells. Arrowheads in (D) and (E) point to cells immunoreactive for both Pax6 and NeuN. At E16, most cells are only immunoreactive for Pax6 (D). However, by E18 (F), all Pax6 immunoreactive cells within the posterior lobe of the cerebellum are also immunoreactive for NeuN. Scale bar in (A) is 250 µm; (D) is 125 µm. The scale for panel D also applies to (B~F).
Acknowledgements
This work was supported by Korean Research Foundation Grant (KRF-2008060.2) funded by the Korean Government.
References
1. Altman J. Postnatal development of the cerebellar cortex in rat.I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972. 145:353–397.
2. Altman J, Bayer SA. Embryonic development of the rat cerebellum. II. Translocation and regional distribution of the deep neurons. J Comp Neurol. 1985. 231:27–41.
3. Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure, and functions. 1997. CRC Press;Chapter 9 and 10.
4. Beierbach E. Lobulation in Mouse Cerebellum. 2001. University of Calgary, Canada: Masters thesis.
5. Beimesche S, Neubauer A, Herzig S, et al. Tissue-specific transcriptional activity of a pancreatic islet cell-specific enhancer sequence/Pax6-binding site determined in normal adult tissues in vivo using transgenic mice. Mol Endocrinol. 1999. 13:718–728.
6. Engelkamp D, Rashbass P, Seawright A, van Heyningen V. Role of Pax6 in development of the cerebellar system. Development. 1999. 126:3585–3596.
7. Hanson I, Van Heyningen V. Pax6: more than meets the eye. Trends Genet. 1995. 11:268–272.
8. Hanson IM, Seawright A, Hardman K, et al. Pax6 mutations in aniridia. Hum Mol Genet. 1993. 2:915–920.
9. Hill RE, Favor J, Hogan BL, et al. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991. 354:522–525.
10. Jensen P, Smeyne R, Goldowitz D. Analysis of cerebellar development in math1 null embryos and chimeras. J Neurosci. 2004. 24:2202–2211.
11. Karam SD, Kim YS, Bothwell M. Granule cells migrate within raphes in the developing cerebellum: an evolutionarily conserved morphogenic event. J Comp Neurol. 2001. 440:127–135.
12. Kawano H, Fukuda T, Kubo K, et al. Pax-6 is required for thalamocortical pathway formation in fetal rats. J Comp Neurol. 1999. 408:147–160.
13. Luckner R, Obst-Pernberg K, Hirano S, Suzuki ST, Redies C. Granule cell raphes in the developing mouse cerebellum. Cell Tissue Res. 2001. 303:159–172.
14. Mastick GS, Davis NM, Andrew GL, Easter SS Jr. Pax6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development. 1997. 124:1985–1997.
15. Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999. 41:281–294.
16. Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988. 457:44–52.
17. Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003. 73:400–409.
18. Yamasaki T, Kawaji K, Ono K, et al. Pax6 regulates granule cell polarization during parallel fiber formation in the developing cerebellum. Development. 2001. 128:3133–3144.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download