Abstract
Purpose
Susceptibility vessel sign (SVS) on gradient echo image, which is caused by MR signal loss due to arterial thrombosis, has been reported in acute middle cerebral artery (MCA) infarction. However, the reported sensitivity and diagnostic accuracy of SVS have been variable. Susceptibility-weighted imaging (SWI) is a newly developed MR sequence. Recent studies have found that SWI may be useful in the field of cerebrovascular diseases, especially for detecting the presence of prominent veins, microbleeds and the SVS. The purpose of this study was to evaluate the diagnostic values of SWI for the detection of hyperacute MCA occlusion.
Materials and Methods
Sixty-nine patients (37 males, 32 females; 46-89 years old [mean, 69.1]) with acute stroke involving the MCA territory underwent MR imaging within 6 hours after the symptom onset. MR examination included T2, FLAIR (fluid-attenuated inversion recovery), DWI, SWI, PWI (perfusion-weighted imaging), contrast-enhanced MR angiography (MRA) and contrast-enhanced T1. Of these patients, 28 patients also underwent digital subtraction angiography (DSA) within 2 hours after MR examination. Presence or absence of SVS on SWI was assessed without knowledge of clinical, DSA and other MR imaging findings.
Susceptibility vessel sign (SVS) on gradient echo (GRE) T2*-weighted image, which is caused by MR signal loss due to arterial thrombus, has been reported in acute middle cerebral artery (MCA) infarction (12). However, the reported sensitivity of SVS for the detection of acute MCA occlusion have been variable (23).
Susceptibility-weighted imaging (SWI) is a recent MR technique, which shows high sensitivity for the detection of paramagnetic substances such as deoxygenated blood, blood products, iron, and calcium (45). Recent studies have found that SWI might be useful in the area of cerebrovascular diseases, especially for the detection and demonstration of the prominent veins, hemorrhage and SVS (6). However, for the detection of acute MCA occlusion, the SVS on SWI has not been fully evaluated.
The purpose of this study was to evaluate the diagnostic values of SWI for the detection of hyperacute MCA occlusion.
This retrospective study was approved by our institutional review board. Informed consent was waived. From November 2011 to July 2012, patients with suspected acute cerebral infarction underwent MR imaging within 6 hours after the onset of symptoms, according to the acute stroke protocol of our institution. The following criteria were established for inclusion in the study: no evidence of intracranial hemorrhage, presence of acute infarction within the MCA territory detected on diffusion-weighted imaging (DWI), and assessment of patency or occlusion of the MCA on contrast-enhanced (CE) MR angiography (MRA). Sixty-nine patients were included in this study (Table 1). There were 37 males and 32 females, aged from 46 to 89 years (mean, 69.1 years). Of these patients, 28 patients also underwent digital subtraction angiography (DSA), as they were thought to possibly benefit from intra-arterial thrombolytic therapy. Severity of the stroke was assessed by using the National Institutes of Health Stroke Scale (NIHSS) on admission. Stroke subtypes were classified according to the TOAST classification (7).
All MR examinations were performed at a 1.5 Tesla MR scanner (Magnetom Avanto; Siemens Medical solutions, Erlangen, Germany). Our stroke protocol consisted of DWI, T2 fluid-attenuated inversion recovery (FLAIR) imaging, fast spin echo (SE) T2-weighted imaging, SWI, CE MRA of the whole head and neck including major intracranial arteries, dynamic susceptibility contrast (DSC) perfusion-weighted imaging (PWI), and CE SE T1-weighted imaging, respectively (Table 2).
SWI was acquired by using a fully velocity-compensated (with gradient moment nulling in all three orthogonal directions), three-dimensional, GRE sequence with the following parameters: repetition time (TR) = 48 ms, echo time (TE) = 40 ms, flip angle (FA) = 15°, bandwidth = 80 kHz, slice thickness = 2 mm, with 64 slices in a single slab, matrix size = 256 × 168. The acquisition time was 3 minutes and 7 seconds with the use of iPAT factor-2. All images were obtained in the same axial plane. Subsequently, 2-mm minimum intensity projection (minIP) images were generated. The sequence, along with entire image processing, was automated on Siemens MR scanner platforms. The phase, magnitude, SWI and minIP images were uploaded and made available on the picture archiving and communication (PACS) system.
CE MRA of the entire neck and major intracranial arteries was performed in the coronal plane by using a fast-spoiled GRE sequence (Table 2). After the acquisition of precontrast scans, a bolus of 0.1 mmol of gadobutrol (Gadovist; Schering, Berlin, Germany) per kilogram of body weight was injected into the antecubital vein by using a power injector (Spectris; Medrad, Pittsburgh, PA, USA) with an injection speed of 1.5 mL/sec, followed by a 20-mL saline flush at the same flow rate. The scans of enhanced MRA were started, when the bolus tract of the contrast media arrived in the carotid artery from the aortic arch. After the subtraction process of precontrast and CE MRA source data, CE MRA images were reconstructed by using a maximum intensity projection (MIP) technique.
In 28 patients, intra-arterial (IA) DSA was performed transfemorally by using a biplane DSA system (Artis Zee; Siemens Medical Solutions, Erlangen, Germany). Bilateral common carotid artery (CCA) or internal carotid artery (ICA) injections were performed. For each vessel, anterior-posterior and lateral projection views were obtained by injection of 8-10 mL of nonionic contrast material (Visipaque 320; Amersham Health, Oslo, Norway). Time interval between MR and DSA examinations was 20 minutes-2 hours (mean, 45 minutes).
All images were retrospectively reviewed. SWIs were independently reviewed by two radiologists who were blinded to the clinical, other MR imaging and DSA findings. Presence or absence of SVS in the MCA was assessed. The SVS on SWI was defined as a blooming artifact within the MCA, which exceeded the size of the homologous contralateral arterial diameter (Fig. 1). In cases of discrepancy, two reviewers reached a consensus later. MRA and DSA images were retrospectively reviewed by an experienced neurointerventional radiologist. The presence and location of the intracranial ICA and/or MCA occlusion were assessed.
It took the average of 2 hours 30 minutes for the time from the stroke onset to MR imaging (range, 42 minutes-6 hours). Initial NIHSS at admission was 0-29 (mean, 11). According to the TOAST classification, large artery atherosclerosis and cardioembolism were 9 (13.0%) and 31 (44.9%), respectively, and 16 patients (23.2%) showed undetermined etiology (Table 1). Of 69 patients, DSA or MRA showed MCA occlusion related to the clinically affected hemisphere in 34 patients (49.3%). The occlusion sites were the distal ICA to MCA (n = 5), MCA M1 (n = 17), and MCA M2 (n = 12), respectively. Among 34 patients who had MCA occlusion on DSA or MRA, 30 (88.2%) showed the SVS on SWI. In all cases, the SVS was demonstrated within the affected MCA (Figs. 1, 2, 3, 4). Four patients showed a false-negative SVS (Fig. 5). One patient showed a false-positive SVS. With DSA or MRA findings as a reference of the gold standard, for the detection of MCA occlusion, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of SVS on SWI were 88.2%, 97.1%, 96.8% and 89.5%, and 92.8%, respectively (Table 3).
MR imaging plays a crucial role in the early detection of cerebral infarction and differentiation of cerebral infarction from other brain pathology which can mimic stroke and it's also useful for the treatment decision in patients with acute infarction (6891011).
In the clinical practice, it is important to determine the presence of vascular occlusion for the evaluation of patients with suspected acute infarction. In case of the major vascular occlusion, accurate assessment of the occlusion site and extent is useful for the decision of treatment strategy. Time-of-flight (TOF) MRA has been widely used for the evaluation of intracranial arterial stenosis or occlusion in patients with cerebral infarction. However, TOF MRA has several limitations. First, although TOF MRA can give high resolution angiographic images, it takes longer acquisition time compared to CE MRA, which can be an obstacle for the patients in unstable condition such as acute stroke. Second, it is insensitive to slow flow and tends to overestimate the stenotic degree. When there is a severe stenosis with the slow distal flow, it can be shown as complete occlusion on TOF MRA. Furthermore, TOF MRA does not always show the correct lesion location and character of the arterial occlusion, which is also caused by signal loss due to decreased flow velocity in the proximal portion of the occlusion site (12). Although CE MRA gives angiographic images of lower spatial resolution compared to TOF MRA, it has several advantages that it can rapidly scan the entire head and neck and major intracranial arteries and it is sensitive to slow flow. As CT angiography and DSA, we can more accurately assess occlusion segment when the distal portion of the occlusion site is reconstituted via collateral pathway, which is important for the consideration of IA thrombolysis (1314). In our MR stroke protocol, we only performed CE MRA for the purpose of rapid IA thrombectomy decision because we thought that CE MRA of the whole head and neck was sufficient to determine the presence of the major vessel occlusion.
The treatment strategy of acute infarction is early recanalization of the occluded vessels in case of major arterial occlusion. With the development of new thrombectomy devices and technical advances, there has been great improvement in the recanalization rate as sell as clinical outcome of acute stroke patients due to major vessel occlusion (15). Because the clinical outcome of the patients depends on the recanalization rate and time from the symptom onset, we have to overcome the time delay between the admission of the patients and starting of thrombolysis (door to needle time). Our stroke MR protocol has been optimized to reduce the time delay due to imaging. In most cases, MR images including MRA and PWI were obtained between 15 minutes.
The SVS is defined as the presence of hypointensity within the vessel lumen on GRE T2*-weighted image. The hypointensity often exceeds the parent vessel diameter due to "blooming" artifact (25). This artifact is caused by severe T2 shortening of high concentrations of deoxyhemoglobin within the clot and less likely due to high hematocrit and hemoglobin as a result of clot retraction and fibrin polymerization (11). SWI is a high-resolution three-dimensional GRE sequence that exploits the magnetic susceptibility differences of the deoxygenated blood and blood products. It is more sensitive for the detection of susceptibility effects than the conventional GRE sequence (161718).
In our study, SWI showed high sensitivity (88.2%), specificity (97.1%) and diagnostic accuracy (92.8%) for the detection of acute MCA occlusion. Among 34 patients with MCA occlusion, SVS was demonstrated in 30 on SWI. Our results were similar to those of recent studies, in which SWI showed high sensitivity (75-93%) and specificity (90%) for the detection of the major intracranial arterial occlusion in acute stroke patients (192021).
For the consideration of mechanical thrombectomy in patients with acute major vessel occlusion, the accurate assessment of occlusion segments and thrombus burden is important for the decision of treatment strategy. The recanalization rate of thrombectomy is also influenced by those factors (222324).
Although SWI can more sensitively detect acute MCA occlusion than conventional GRE technique, it also shows great susceptibility artifact in area of the skull base. In the present study, there were some difficulties for the accurate assessment of proximal extent in cases of combined distal ICA occlusion.
Our study had several limitations. First, it was a retrospective study which had the possibility of a sampling bias. Second, the number of patients was relatively small. Further studies with large sample size are needed for the validation of SWI in acute MCA occlusion. Third, long-term follow-up data for assessing the prognostic value of the SVS are lacking in our study. Fourth, DSA was not performed in all patients of our study and it has been a gold standard until now. However, DSA is an invasive tool having potential risks. In a recent study of comparison with DSA, CE MRA also proved reliable for identifying occlusion location in acute stroke (14).
In conclusion, SWI was sensitive, specific and accurate for the detection of hyperacute MCA occlusion and it might also be helpful for the treatment decision such as mechanical thrombectomy.
Figures and Tables
Fig. 1
A 62-year-old female patient with right hemiparesis and aphasia, 2 hours ago. (a) DWI image shows acute infarction in the left frontal lobe. (b) SWI reveals a SVS sign in the left MCA M2 segment (arrow). (c) On MRA, the left MCA M2 segment is completely occluded (arrow). DWI = diffusion-weighted imaging; MCA = middle cerebral artery; MRA = MR angiography; SVS = susceptibility vessel sign; SWI = susceptibility-weighted imaging
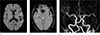
Fig. 2
A 62-year-old male with right hemiparesis and aphasia, 2.5 hours ago. (a) DWI shows hyperintense lesion in the left insula. (b) SWI reveals a SVS sign in the proximal M2 segment of the left MCA (arrows). (c, d) On MRA (c) and DSA (d) images, M2 segment of the left MCA is completely occluded (arrows). DSA = digital subtraction angiography; DWI = diffusion-weighted imaging; MCA = middle cerebral artery; MRA = MR angiography; SWI = susceptibility-weighted imagin
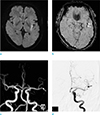
Fig. 3
A 74-year-old male with right hemiplegia and aphasia, 3.5 hours ago. (a) DWI shows hyperintense lesions in the left MCA territory. (b) SWI reveals a SVS sign in the distal M1 to proximal M2 segment of the left MCA (arrow). (c-e) MRA (c) and DSA (d, e) images show segmental occlusion of the proximal to cavernous ICA. The distal ICA is reconstituted via collaterals from the ECA (small arrows). There is also complete occlusion in the distal M1 segment of the left MCA (arrow). DSA = digital subtraction angiography; DWI = diffusion-weighted imaging; ECA = external carotid artery; ICA = internal carotid artery; MCA = middle cerebral artery; MRA = MR angiography; SVS = susceptibility vessel sign; SWI = susceptibility-weighted imaging
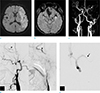
Fig. 4
A 74-year-old female with right hemiplegia and aphasia, 2 hours ago. (a) DWI shows hyperintense lesion in the entire left cerebral hemisphere. (b) SWI reveals a SVS sign in the left distal ICA to MCA M1-2 segment (arrows). (c, d) MRA images show complete occlusion of the left CCA, ICA, ACA and MCA. ACA = anterior cerebral artery; CCA = common carotid artery; DWI = diffusion-weighted imaging; ICA = internal carotid artery; MCA = middle cerebral artery; MRA = MR angiography; SVS = susceptibility vessel sign; SWI = susceptibility-weighted imaging
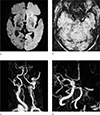
Fig. 5
84-year-old male with right hemiplegia and aphasia, 2 hours ago. (a) DWI shows hyperintense lesion in the left MCA territory. (b) SWI reveals no definite SVS sign. (c, d) MRA (c) and DSA (d) images show complete occlusion in the proximal M1 segment of the left MCA (arrows). DSA = digital subtraction angiography; DWI = diffusion-weighted imaging; MCA = middle cerebral artery; MRA = MR angiography; SVS = susceptibility vessel sign; SWI = susceptibility-weighted imaging
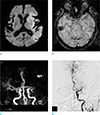
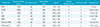
Table 1
Descriptive Statistics of the Patients

Table 2
MR Imaging Protocol
References
1. Flacke S, Urbach H, Keller E, et al. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: clinical importance and comparison with hyperdense MCA sign at CT. Radiology. 2000; 215:476–482.
2. Rovira A, Orellana P, Alvarez-Sabin J, et al. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology. 2004; 232:466–473.
3. Adams HP Jr, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003; 34:1056–1083.
4. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004; 52:612–618.
5. Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009; 64:74–83.
6. Huang P, Chen CH, Lin WC, Lin RT, Khor GT, Liu CK. Clinical applications of susceptibility weighted imaging in patients with major stroke. J Neurol. 2012; 259:1426–1432.
7. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.
8. Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. 2005; 36:2379–2383.
9. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009; 30:19–30.
10. Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009; 30:232–252.
11. Hermier M, Nighoghossian N. Contribution of susceptibility-weighted imaging to acute stroke assessment. Stroke. 2004; 35:1989–1994.
12. Ishimaru H, Ochi M, Morikawa M, et al. Accuracy of pre- and postcontrast 3D time-of-flight MR angiography in patients with acute ischemic stroke: correlation with catheter angiography. AJNR Am J Neuroradiol. 2007; 28:923–926.
13. Kinoshita T, Ogawa T, Kado H, Sasaki N, Okudera T. CT angiography in the evaluation of intracranial occlusive disease with collateral circulation: comparison with MR angiography. Clin Imaging. 2005; 29:303–306.
14. Le Bras A, Raoult H, Ferre JC, Ronziere T, Gauvrit JY. Optimal MRI sequence for identifying occlusion location in acute stroke: which value of time-resolved contrast-enhanced MRA. AJNR Am J Neuroradiol. 2015; 36:1081–1088.
15. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20.
16. Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997; 204:272–277.
17. Rauscher A, Sedlacik J, Barth M, Mentzel HJ, Reichenbach JR. Magnetic susceptibility-weighted MR phase imaging of the human brain. AJNR Am J Neuroradiol. 2005; 26:736–742.
18. Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009; 29:1433–1449.
19. Radbruch A, Mucke J, Schweser F, et al. Comparison of susceptibility weighted imaging and TOF-angiography for the detection of Thrombi in acute stroke. PLoS One. 2013; 8:e63459.
20. Park MG, Oh SJ, Baik SK, Jung DS, Park KP. Susceptibility-weighted imaging for detection of thrombus in acute cardioembolic stroke. J Stroke. 2016; 18:73–79.
21. Agarwal A, Vijay K, Thamburaj K, Kanekar S, Kalapos P. Sensitivity of 3D gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography angiography for detection of middle cerebral artery thrombus in acute stroke. Neurol Int. 2014; 6:5521.
22. Kim HS, Lee DH, Choi CG, Kim SJ, Suh DC. Progression of middle cerebral artery susceptibility sign on T2*-weighted images: its effect on recanalization and clinical outcome after thrombolysis. AJR Am J Roentgenol. 2006; 187:W650–W657.
23. Rohan V, Baxa J, Tupy R, et al. Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke. 2014; 45:2010–2017.
24. Soize S, Batista AL, Rodriguez Regent C, et al. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol. 2015; 22:967–972.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download