ABSTRACT
Background:
4-1BB mediated costimulatory signal is a recently identified immunotherapeutic strategy for treating autoimmune diseases without depressing the immune response. In this study, we investigated the expression of 4-1BB and 4-1BBL on the peripheral blood mononuclear cells (PBMC) and we assessed whether the serum levels of soluble (s) 4-1BB and s4-1BBL in the patients with Graves’ disease (GD) and compared them with normal subjects.
Methods:
Expression of 4-1BB and 4-1BBL on PBMC of GD patients was determined by flow cytometry. The concentrations of s4-1BB and s4-1BBL were assessed in the sera of GD patients by performing ELISA.
Results:
4-1BB was constitutively expressed on naive CD4+ and CD8+ T cells of the GD patients and this was increased by stimulation. 4-1BBL was also expressed on the antigen-presenting cells such as CD19+ B cells, monocytes and dendritic cells in GD patients. The serum levels of s4-1BB and s4-1BBL were significantly higher in GD patients than those in controls, and these levels were significantly correlated with the serum levels of thyroid-binding inhibitory immunoglobulin and free T4.
Conclusion:
These results indicate that effector T cells of GD patients can be activated through the 4-1BB-mediated costimulatory signal. Elevated s4-1BB and s4-1BBL levels in the sera of GD patients may affect modulation of the clinical course in GD patients. (J Kor Soc Endocrinol 21:116~124, 2006)
References
2. Heufelder AE. Pathogenesis of ophthalmopathy in autoimmune thyroid disease. Rev Endocr Metab Disord. 2000; 1:87–95.
3. Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003; 148:1–9.

4. Salmaso C, Olive D, Pesce G, Bagnasco M. Costimulatory molecules and autoimmune thyroid diseases. Autoimmunity. 2002; 35:159–167.

5. Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003; 14:265–273.

6. Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002; 27:19–26.

7. Kwon B, Yu KY, Ni J, Yu GL, Jang IK, Kim YJ, Xing L, Liu D, Wang SX, Kwon BS. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J Biol Chem. 1999; 274:6056–6061.

8. Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med. 1999; 190:1535–1540.

9. Mittler RS, Foell J, McCausland M, Strahotin S, Niu L, Bapat L, Hewes LB. Anti-CD137 antibodies in the treatment of autoimmune disease and cancer. Immunol Res. 2004; 29:197–208.

10. Foell J, Strahotin S, O’Neil SP, McCausland MM, Suwyn C, Haber M, Chander PN, Bapat AS, Yan XJ, Chiorazzi M, Hoffmann MK, Mittler RS. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest. 2003; 111:1505–1518.
11. Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu XY. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002; 168:1457–1465.

12. Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004; 10:1088–1094.

13. Chistiakov DA, Turakulov RI. CTLA-4 and its role in autoimmune thyroid disease. J Mol Endocrinol. 2003; 31:21–36.

14. Anand A, Dean GS, Quereshi K, Isenberg DA, Lydyard PM. Characterization of CD3+CD4-CD8-(double negative) T cells in patients with systemic lupus erythematosus: activation markers. Lupus. 2002; 11:493–500.
15. Brunner-Weinzierl MC, Hoff H, Burmester GR. Multiple functions for CD28 and cytotoxic T lymphocyte antigen-4 during different phases of T cell responses: implications for arthritis and autoimmune diseases. Arthritis Res Ther. 2004; 6:45–54.
16. Nakamoto Y, Niki M, Watanabe M, Iwatani Y. Increase in immunoglobulin G3-secreting cells in intractable Graves’ disease. Thyroid. 2003; 13:325–331.

17. Watanabe M, Yamamoto N, Matsuzuka F, Miyauchi A, Iwatani Y. Decrease of CD154 intensity on peripheral CD4+ T cells in autoimmune thyroid disease. Clin Exp Immunol. 2004; 136:555–558.

18. Metkar SS, Naresh KN, Manna PP, Srinivas V, Advani SH, Nadkarni JJ. Circulating levels of TNF alpha and TNF receptor superfamily members in lymphoid neoplasia. Am J Hematol. 2000; 65:105–110.
19. Setareh M, Schwarz H, Lotz M. A mRNA variant encoding a soluble form of 4-1BB, a member of the murine NGF/TNF receptor family. Gene. 1995; 164:311–315.

20. Michel J, Schwarz H. Expression of soluble CD137 correlates with activation-induced cell death of lymphocytes. Cytokine. 2000; 12:742–746.

21. Salih HR, Schmetzer HM, Burke C, Starling GC, Dunn R, Pelka-Fleischer R, Nuessler V, Kiener PA. Soluble CD137 (4-1BB) ligand is released following leukocyte activation and is found in sera of patients with hematological malignancies. J Immunol. 2001; 167:4059–4066.

22. Salih HR, Nuessler V, Denzlinger C, Starling GC, Kiener PA, Schmetzer HM. Serum levels of CD137 ligand and CD178 are prognostic factors for progression of myelodysplastic syndrome. Leuk Lymphoma. 2004; 45:301–308.

23. Michel J, Langstein J, Hofstadter F, Schwarz H. A soluble form of CD137 (ILA/4-1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol. 1998; 28:290–295.

24. Jung HW, Choi SW, Choi JI, Kwon BS. Serum concentrations of soluble 4-1BB and4-1BB ligand correlated with the disease severity in rheumatoid arthritis. Exp Mol Med. 2004; 36:13–22.
Fig. 1.
Expression levels of 4-1BB and 4-1BBL on PBMC of the patients with Graves’ disease (GD). Naïve PBMC were activated with 1 μg/mL of anti-CD3 mAb and 5 μg/mL of LPS for 24 hr. Expression levels of 4-1BB (A) and 4-1BBL (B) on PBMC of patients with GD were analyzed by flow cytometry compared with control group.
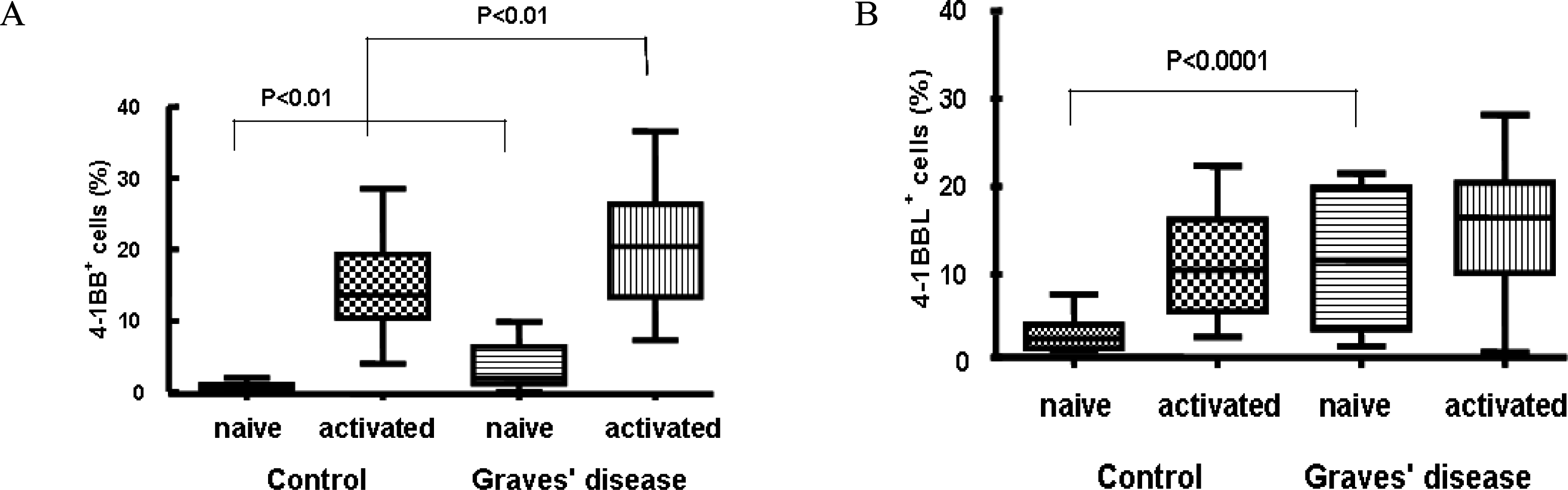
Fig. 2.
Expression patterns of 4-1BB and 4-1BBL on PBMC of the patients with Graves’ disease. A and C, PE conjugated CD4+/CD8+ T cells with FITC conjugated 4-1BB/4-1BBL fluorescence dot plots in the lymphocytes population of PBMC with healthy subject (A) and GD patient (C) by two-color cytometry. Square box presents double-positive cell population. B and D, 4-1BBL expression on primary monocytes (Mo) and monocyte-derived dendritic cells (DCs) of healthy subject (B) and GD patient (D) by single-color flow cytometric histogram. The thick lines represent 4-1BBL+ cells and the thin lines represent isotype-matched mAb as a control.
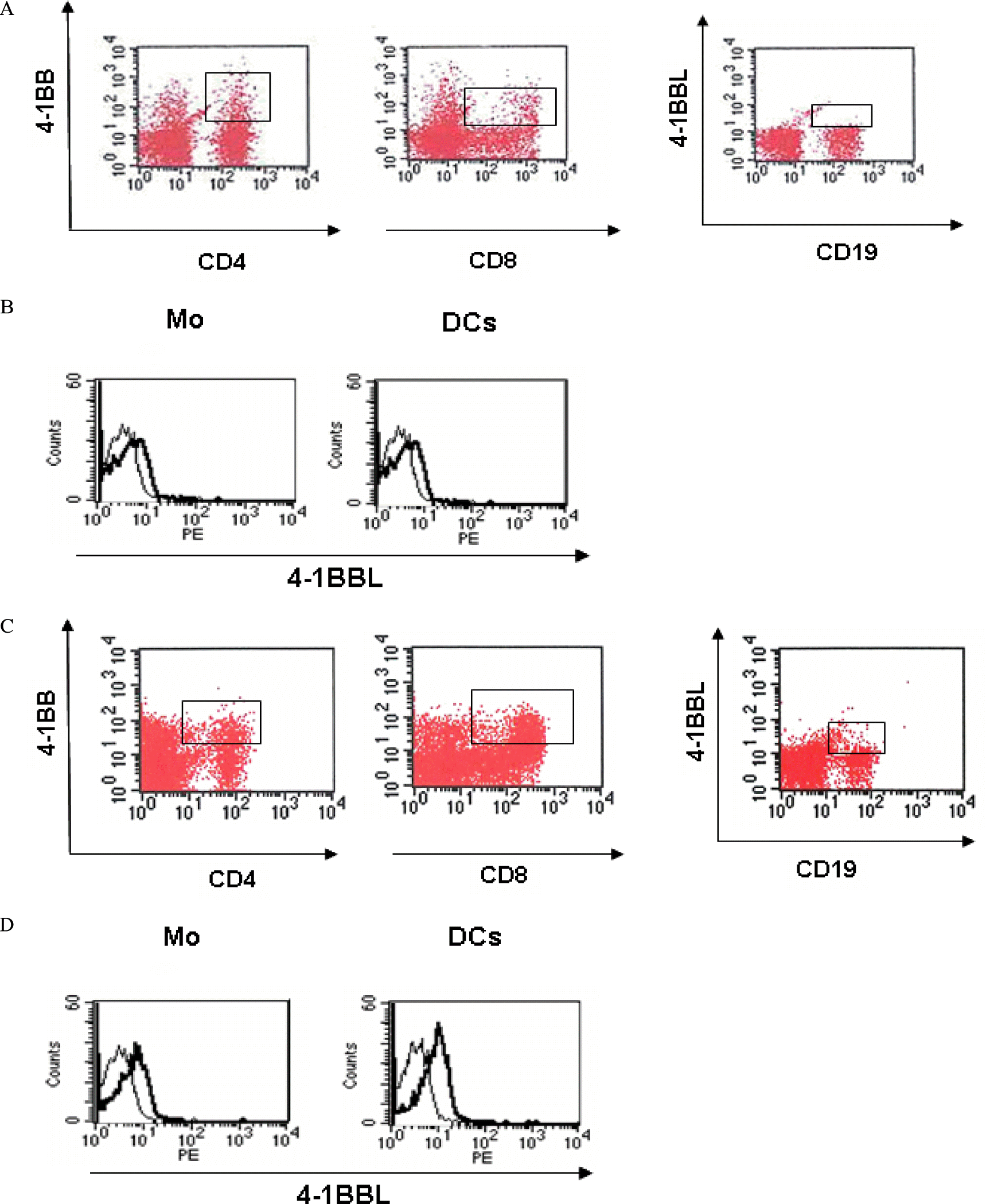
Fig. 3.
Serum levels of s4-1BB and s4-1BBL in the patients with Graves’ disease. s4-1BB (A) and s4-1BBL (B) were assessed in sera of the patients with Graves’ disease by ELISA and compared with normal subjects as a control.
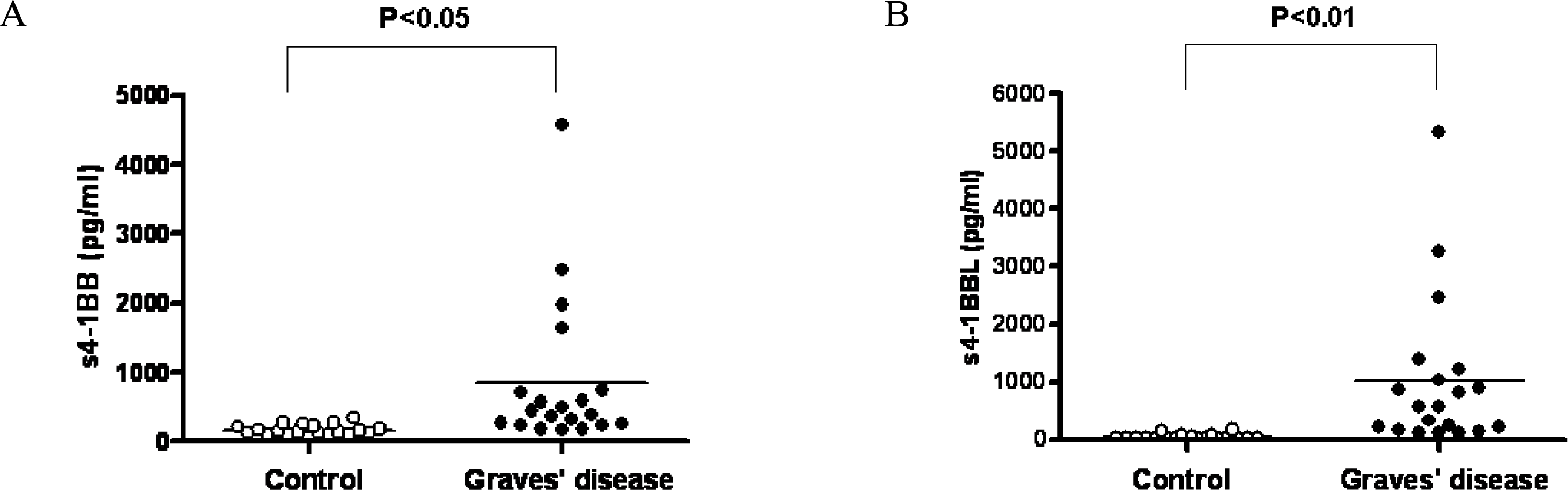
Fig. 4.
Correlation between s4-1BB and s4-1BBL levels in sera of the patients with Graves’ disease. The concentrations of s4-1BB and s4-1BBL were measured in sera of the same patients by ELISA.
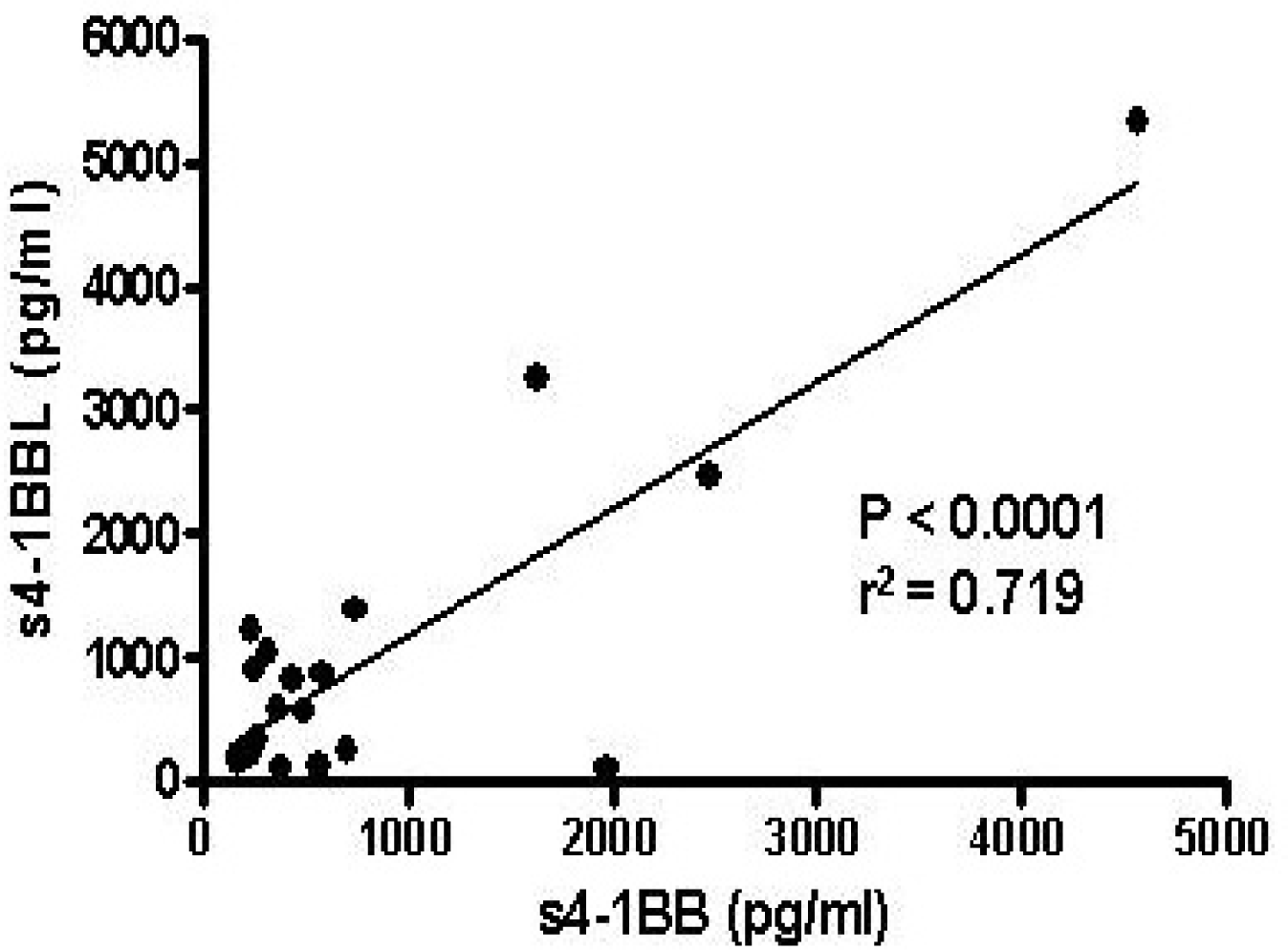
Fig. 5.
Correlation between s4-1BB/s4-1BBL and clinical parameters of the patients with Graves’ disease (GD). A, Correlation between s4-1BB and TBII (%) levels in sera of the patients with GD. B, Correlation between s4-1BB and free T4 (ng/mL) levels in sera of GD patients. C, Correlation between s4-1BBL and TBII levels in sera of GD patients. D, Correlation between s4-1BBL and free T4 levels in sera of GD patients.
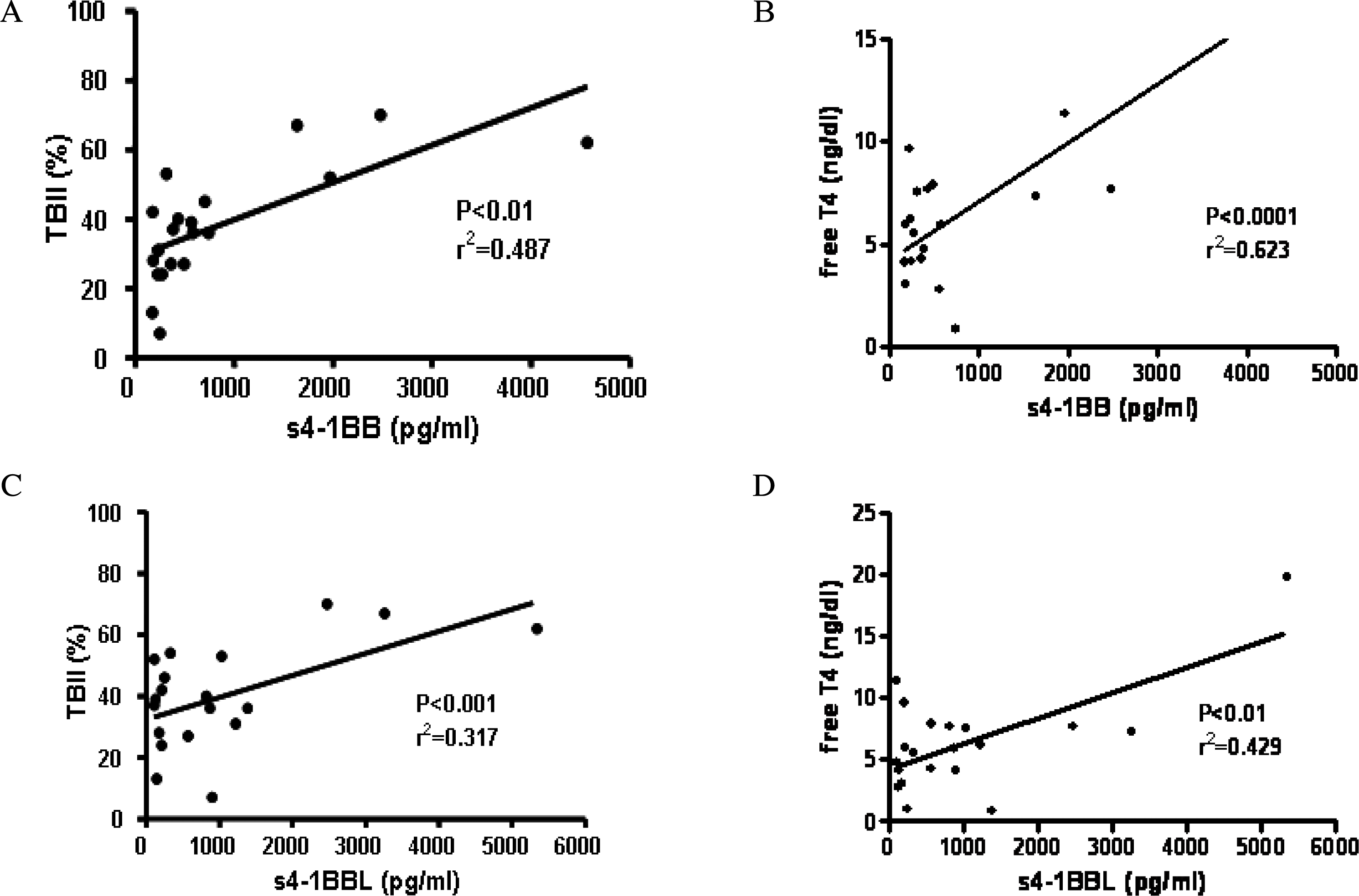
Table 1.
Clinical characteristics of each study group
|
Age (years) |
Gender |
TBII (%) |
free T4 (ng/dL) |
TSH (μU/mL) |
TPOAb (IU/mL) |
TgAb (IU/mL) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Value | N | F | M | 0-15.0 | 0.89-1.81 | 0.35-5.50 | 0-25 | 0-40 | |
| Graves’ | Disease | 20 | 36 ± 11 | 16 | 4 | 40.9 ± 17.3* | 6.43 ±2.39* | 0.01 ± 0.00* | 466 ±358* | 304 ±683 |
| Control | 20 | 42 ± 8 | 20 | 0 | 3.8 ± 2.1 | 1.4 ±1.06 | 0.58 ± 0.68 | 12 ±11.3 | 2.6 ±3.4 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download