Abstract
Backgrounds
L-type voltage-dependent calcium channel (LTCC) plays a crucial role in insulin secretion from pancreatic β cells through Ca2+ influx. In the recent report, LTCC Cav1.3 subtype homozygous knock out mice showed impairment of postnatal pancreatic beta cell development as well as insulin secretion.
Methods
We performed 90% partial pancreatectomy in heterozygous Cav1.3 knock out mice to investigate the effect of partial deficiency of Cav1.3 gene on beta cell regeneration in the adult. Glucose homeostasis, metabolic profiles including serum insulin and lipid levels and morphologic changes of pancreatic islets were studied.
Results
90% Partial pancreatectomy induced glucose intolerance only in the heterozygous knock out mice at 8 weeks after surgery. Distribution of islet size was significantly different between two groups after partial pancreatectomy; median value of islet size of heterozygote was larger than that of wild type (642.8 µm2 vs 1459.8 µm2,P < 0.01). The frequency of single beta cell unit, considered as a unit of β cell neogenesis, was much lower in heterozygote than that of wild type (41% vs 23.3%, P < 0.05).
Figures and Tables
Fig. 1
α1D genotyping of animals. PCR of tail DNA showed wild type (+/+) and heterozygote (+/-). C, normal C57BL/6J mouse used as control animal; SM, size marker; NC, negative control of PCR.
Fig. 2
Comparison of changes in body weight and fed whole blood glucose levels after partial pancreatectomy between wild type and heterozygote. A, Body weights temporarily decreased after operation and then gradually increased throughout experiment in both groups. B, Whole blood glucose levels were measured at fed state in the morning with portable glucometer weekly. Blood glucose levels increased one week after operation in both groups and maintained in HT and gradually decreased after 3 weeks in WT. WT, wild type; HT, heterozygote. *P < 0.05.

Fig. 3
Comparison of baseline IPGTT and ITT between wild type and heterozygote. A, Glucose tolerance test before pancreatectomy showed no significant differences between two groups. WT, wild type, n = 14; HT, heterozygote, n = 39. B, Glucose disappearance rate after insulin injection showed no significant differences between groups. WT, n = 12; HT, n = 11. IPGTT, intraperitoneal glucose tolerance test; ITT, insulin tolerance test.

Fig. 4
Comparison of IPGTT and ITT performed 8 weeks after partial pancreatectomy between wild type and heterozygote. A, Blood glucose levels during glucose tolerance test after pancreatectomy in WT and HT groups. Blood glucose level of HT at ninety minute during GTT was significantly higher than that of WT. In each group, n = 5. B, Glucose disappearance rates after insulin injection were not different between groups. In each group, n = 5. WT, wild type; HT, heterozygote. IPGTT, intraperitoneal glucose tolerance test; ITT, insulin tolerance test. *P < 0.05.

Fig. 5
Median value of islet size in wild type and heterozygote groups. n = 5 in wild type (WT), n = 6 in heterozygote (HT). *P < 0.05.
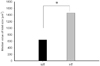
Fig. 6
Islet size distribution in wild type and heterozygote groups. Frequency of SCU (single cell unit), SI (small islet), MI (medium-sized islet), and LI (large-sized islet) in both groups. Proportion of SCU was less in HT than WT. Size of islets in HT group has a tendency to be larger than that of WT. n = 5 in wild type (WT), n = 6 in heterozygote (HT). *P < 0.05.

Fig. 7
Immunohistochemical stain of islet with anti-insulin antibody. Relatively larger sized islets were observed in the heterozygote (HT) (b,d) than wild type (WT) (a, c).
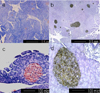
Table 1
Biochemical and hormonal parameters of wild type (WT) and heterozygote (HT) of α1D Knock out mice before, 3 days and 8 weeks after partial pancreatectomy

Table 2
Total pancreatic weight and beta cell mass in remnant pancreatic tissue of wild type (WT) and heterozygote (HT) of α1D knock out mice before and 3 days, 8 weeks after 90% partial pancreatectomy

References
1. Kahn SE. The importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001. 86:4047–4058.
2. Schulla V, Renström E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundauist I, Magnson MA, Obermüller S, Olofsson C, Salehi A, Wendt A, Klugbauer N, Wolheim CB, Rorsman P, Hofmann F. Impaired insulin secretion and glucose tolerance in β cell-selective Cav1.2 Ca2+ channel null mice. EMBO. 2003. 22:3844–3854.
3. Smith PA, Rorsmann P, Ashcroft FM. Modulation of dihydropyridine-sensitive Ca2+ channels by glucose metabolism in mouse pancreatic beta-cells. Nature. 1989. 342:550–553.
4. Ashcroft FM, Proks P, Smith PA, Ämmälä C, Bokvist K, Rorsman P. Stimulus-secretion coupling in pancreatic β cells. J Cell Biochem. 1994. 55S. 54–65.
5. Sher E, Giovnnini F, Codignola A, Passafaro M, Giorgi-Rossi P, Volsen S, Craig P, Davalli A, Carrera P. Voltage-operated calcium channel heterogeneity in pancreatic β cells: physiopathological implications. J Bioenerg Biomemb. 2003. 35:687–696.
6. Seino S, Chen L, Seino M, Blondel O, Takeda J, Johnson JH, Bell GI. Cloning of the α1 subunit of a voltage-dependent calcium channel expressed in pancreatic β cells. Proc Natl Acad Sci. 1992. 94:584–588.
7. Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ. Predeominance of the α1D channels of hair cells in the chicken's cochlea. Proc Natl Acad Sci. 1997. 94:14883–14888.
8. Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perea-Reyes E, Schwarz A, Snutch TP, Tanabe T, Birnboumer L, Tsien TW. Nomenclature of voltage-gated calcium channels. Neuron. 2000. 25:533–535.
9. Yang SN, Larsson O, Bränström R, Bertorello AM, Leibiger B, Leibiger IB, Moede T, Köler M, Meister B, Berggren PO. Syntaxin 1 interacts with the LD subtype of voltage-gated Ca2+ channels in pancreatic β cells. Proc Natl Acad Sci. 1999. 96:10164–10169.
10. Barg S, Xiaosong M, Eliasson L, Galvanovskis J, Göpel SO, Obermüler S, Platzer J, Renström E, Trus M, Atlas D, Striessnig J, Rorsman P. Fast exocytosis with few Ca2+ channels in insulin-secreting mouse pancreatic B cells. Biophys J. 2001. 81:3308–3323.
11. Platzer J, Engel J, Schrott-Fischer A, Stephen K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000. 102:89–97.
12. Namkung Y, Skrypnyk N, Neong MJ, Lee T, Lee MS, Kim HL, Chin H, Suh PG, Kim SS, Shin HS. Requirement for the L-type Ca2+ channel α1D subunit in postnatal β cell generation. J Clin Invest. 2001. 108:1015–1022.
13. Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of the adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes. 1993. 42:1715–1720.
14. Namkung Y, Smith SM, Lee SB, Skrypnyk NV, Kim HL, Chin H, Scheller RH, Tsien RW, Shin HS. Targeted disruption of the Ca2+ channel β3 subunit reduces N- and L-type Ca2+ channel activity and alters the voltage-dependent activation of P/Q-type Ca2+ channels in neurons. Proc Natl Acad Sci. 1998. 95:12010–12015.
15. Cho CH, Kim SS, Jeong MJ, Lee CO, Shin HS. The Na+-Ca2+ exchanger is essential for embryonic heart development in mice. Mol Cell. 2000. 10:712–722.
16. Bonner-Weir S, Trent DF, Weit GC. Partial pancreatectomy and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983. 71:1544–1553.
17. Weibel ER. Principals and methods for the morphometric studies of the lung and other organs. Lab Invest. 1963. 12:131–155.
18. Bonner-Weir S. β cell turnover. Its assessment and implications. Diabetes. 2001. 50:S20–S24.
19. Weibel ER. Stereologic methods. practical methods for biologic morphometry. 1978. Vol. 1. London: Academic press;101–161. .
20. Bouwens L, Pipeleers DG. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia. 1998. 41:629–633.
21. Iwashima Y, Kondoh-Abiko A, Seino S, Takeda J, Eto M, Polonsky KS, Makino I. Reduced levels of messenger ribonucleic acid for calcium channel, glucose transporter-2, and glucokinase are associated with alterations in insulin secretion in fasted rats. Endocrinology. 1994. 135:1010–1017.
22. Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes. 1988. 37:232–236.
23. Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir G. Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. J Biol Chem. 1999. 274:14112–14121.
24. Roe MW, Worley JF III, Tokuyama Y, Philipson LH, Sturis J, Tang J, Dukes ID, Bell GI, Polonsky KS. NIDDM is associated with loss of pancreatic β-cell L-type Ca2+ channel activity. Am J Phsiol. 1996. 270:E133–E140. (Endocrinol Metab 33).
25. Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas: Estimation with simple mathematical model. Diabetes. 1995. 44:249–256.
26. Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999. 48:2270–2276.
27. Lipsett M, Finegood DT. β cell neogenesis during prolonged hyperglycemia in rats. Diabete. 2002. 51:1834–1841.
28. Fajas L, Annicotte JS, Miard S, Sarruf D, Watanabe M, Auwerx J. Impaired pancreatic growth, β cell mass, and β cell function in E2F1-/- mice. J Clin Invest. 2004. 113:1288–1295.
29. Huang H, Chu K, Nemoz-Gaillard E, Elberg D, Tsai MJ. Neogenesis of β cells in adult BETA2/NeuroD deficient mice. Mol Endocrin. 2002. 16:541–561.
30. De Leon D, Deng S, Madani R, Ahima RS, Drucker DJ, Stoffers DA. Role of endogenous Glucagon-Like Peptide-1 in islet regeneration after partial pancreatectomy. Diabetes. 2003. 52:365–371.
31. Permutt MA. Use of DNA polymorphism for genetic analysis of non-insulin dependent diabetes mellitus. Baillieres Clin Endocrinol Metab. 1991. 5:495–526.
32. MaCarthy MI, Froguel P. Genetic approaches to the molecular understanding of type 2 diabetes. Am J Physiol Endocrinol Metab. 2002. 283:E217–E225.
33. Bell GI, Pilkis SJ, Polonsky KS. Glucokinase mutations, insulin secretion, and diabets mellitus. Annu Rev Physiol. 1996. 58:171–186.
34. Yamagata K, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI. Mutations in the hepatocyte nuclear factor-4 gene in maturity-onset diabetes of the young (MODY1). Nature. 1996. 384:458–460.
35. Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI. Mutation in hepatocyte nuclear factor-1β gene (TCF2) associated with MODY. Nature Genet. 1997. 17:384–385.
36. Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nature Genet. 1997. 15:106–110.
37. Bonner-Weir S. Perspective: Postnatal pancreatic β cell growth. Endocrinology. 2000. 141:1926–1929.
38. Tsunoda K, Sanke T, Nakagawa T, Furuta H, Nanjo K. Single nucleotide polymorphism (D68D, T to C) in the syntaxin 1A gene correlates to age at onset and insulin requirement in type II diabetic patients. Diabetologia. 2001. 44:2092–2097.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download