Abstract
Background
Rapeseed-mustard is the second most important source of edible oil in India. Several species of Brassica are grown in different parts of country for its oilseeds.
Objective
The objective was to investigate allergenicity to antigenic extracts of pollen of 4 species of Brassica.
Methods
Brassica campestris, Brassica juncea, Brassica nigra, and Brassica napus were selected for the detailed investigation. Pollen samples from each of the four species were collected from the polliniferous materials. The antigenic and allergenic profiles of these extracts were evaluated by means of sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Skin prick test, enzyme linked immuno sorbent assay and Western blot on atopic individuals.
Results
Out of the 159 atopic subjects tested, 21.38% were positive to at least one or other species of Brassica pollen, with highest skin positivity (13.20%) to B. campestris extract. Raised IgE with significant linear correlation with intensity of skin reactions was obtained. Protein fractions of 20, 25, 32, 37, 56, and 90 kDa were recognized by B. campestris and B. juncea whereas 56, 76, 87, and 90 kDa were recognized by B. nigra and B. napus as major IgE binding protein fractions. The patients also showed positivity to other inhalant pollen allergens tested.
Conclusion
IgE mediated hypersensitivity varied from 4.40% to 13.20% in Indian atopic subjects to pollen of one or the other species of Brassica. Protein fractions of 47, 56, 76, 87, and 90 kDa were identified as IgE binding by all the four species, however individual heterogeneity exists. Thus a local species may be more pertinent for immunotherapy. The major allergen needs to be further characterized.
As early as in 1954, Colldahl [1] ascribed a case of conjunctivitis, rhinitis and asthma by rape pollen. Aerobiological surveys in India have quantitated its aerial pollen incidence of Brassica spp to be 1.5-6.5% of the total annual pollen catch and as high pollen producer [2, 3, 4]. Several workers from United Kingdom (UK) and Europe have examined the potential role of oilseed rape during the last two decades as evident by spurt of publications on the subject [5, 6, 7, 8, 9, 10]. Bugur and Arner [11] reported sensitization in 23% of atopic cases from Scandinavia whereas in Austria, 7.1% of pollen-allergic patients showed sensitization to rape pollen [6]. However from UK, a prevalence of less than 0.2% allergy to oilseed rape pollen has been reported [5]. Due to differences in the degree of sensitization as reported by different groups, it was considered important to get to the bottom of this problem in India.
Backed by the legacy of India's 5,000-year-old civilization, rapeseed-mustard is the second most important source of edible oil in India grown for vegetable, essential oil and condiment. It occupies a sizable share (15.2%) of country's gross cropped area [12]. Of the several species on the Indian subcontinent, Brassica campestris, Brassica juncea, Brassica nigra, and Brassica napus are the most extensively cultivated. Inspite of such extensive cultivation, there has been no systematic evaluation of the relative sensitization pattern of the various species of Brassica in India. Therefore, the present work was aimed at assessing sensitization to pollen of commonly cultivated species in atopic population.
Polliniferous materials from B. campestris, B. juncea, B. nigra, and B. napus were collected during three months of flowering from agricultural plots specifically cultivated for these species at Indian Agricultural Research Institute, Delhi, during December 2004 to May 2005. After drying at 37℃ for few days, pollen were procured by gently crushing heads and passing through different grades of sieves. Pollen purity as per the method of Cour and Loublier [13] was checked and samples with more than 90% purity were used for antigen extraction.
The extraction was carried out at Antigen Production Laboratory of the Institute of Genomics & Integrative Biology as per standard protocol [14]. Briefly, the defatted pollen were suspended (1:20 weight/volume [w/v]) in Ammonium Bi-Carbonate Buffer (pH 7.4), containing 2mM ethylenediaminetetraacetic acid and 1mM phenyl methyl sulfonyl fluoride for overnight at 4℃. The extracts were centrifuged for 20 minutes at 12,000 rpm at 4℃, lyophilized and stored. Protein content was estimated as per Lowry's method with slight modification [14].
Patients from in and around Delhi having respiratory symptoms attending allergy Clinic from January 2005 to September 2006 were included. The inclusion criterion for selection was those patients suffering with either bronchial asthma (BA) or allergic rhinitis (AR) or both as assessed by clinical and hematological parameters at allergy clinics. The Institutional Ethics Committee had approved the study and written informed consent was obtained from each participant. In case of minors, consent was taken from parents. During the first visit, detailed medical questionnaire of the participating subjects were filled, instructions for disuse of antihistamines were given under clinical supervision. Altogether, 194 patients were recruited for investigation but 35 did not participate due to different reasons such as distance from Delhi, illness, lack of interest. Thus, only 159 atopic patients were found eligible for assessment of allergy due to Brassica pollen.
Skin prick test (SPT) were performed on 159 atopic cases according to standard protocol by trained personnel with extracts of pollen of B. campestris, B. juncea, B. nigra, B. napus. and with panel of inhalant allergens comprising of Artemisia scoparia, Amaranthus spinosus, Cannabis sativa, Chenopodium album, Cyanodon dactylon, Holoptelia integrifolia, Imperata cylindrica, Morus alba, Prosopis juliflora, Xanthium strumarium and house dust mite (Dermatophagoides farinae) to find out sensitization in the atopic patients.
Briefly, a drop of the glycerinated extract (50%) was placed on the volar aspect of the forearm, pricked with a 23 G sterile needle and skin was slightly raised to allow antigen to enter. The wheal and flare reactions were graded after 20 minutes. Histamine dihydrochloride (1 mg/mL) was used as positive control and 50% glycerinated buffer as negative control. Skin reactions were graded as per the criteria outlined by Singh et al. [15] and cases showing positivity of 2+ and above were considered as markedly positive. Sera were collected from SPT positive patients and normal healthy volunteers (n = 50) for immunoassays of pollen extracts.
Total serum IgE was estimated with the help of the sandwich enzyme-linked immunosorbent assay (ELISA) technique. Monoclonal antihuman IgE antibody (Bethyl Laboratories Inc., Montgomery, TX, USA) was coated (1µg/well) onto ELISA plate (Nunc Maxisorp, A/S Rocskilde, Denmark) and incubated overnight at 4℃. Washing with phosphate buffered saline (PBS) was carried after each step. IgE calibrators and serum samples diluted (1:10 volume/volume [v/v]) were added to the wells and incubated overnight at 4℃. After repeated washings, detection antibody anti human IgE conjugated to horse radish peroxidase (diluted 1: 10,000 v/v) was added to the plates and incubated at room temperature for 3 hours. The colour reaction was developed with ABTS (2/2/azino bis tetra sulphonic acid), and optical density (OD) was measured at 405 nm using an ELISA reader Spectra Max (Molecular Devices, Sunny Bell, CA, USA). A standard curve was generated using IgE calibrators and was plotted on a Lin-Log graph. All values are expressed in IU/mL (1 IU/mL= 2.4 ng/mL).
Allergen-specific IgE antibodies against pollen of Brassica extracts were measured by ELISA [16]. Briefly, microtitre plates (Maxisorp; Nunc TM Immunomodule, Roskilde, Denmark) were coated with 20-µg protein in 100 µL per well in carbonate buffer (pH 9.6). Nonspecific sites were blocked by 2% bovine serum albumin (BSA), washed and incubated with sera of the individual patients (1:10 v/v). The serum from healthy volunteers was tested as negative controls. After washing, the plates were incubated for 2 hours with antihuman IgE-HRP (1:1000 v/v; Sigma Chemical Co., St Louis, MO, USA) in PBS. Colour was developed and the absorbance was read at 492 nm. Percent binding was calculated for each sample relative to control sera as follows:
Sera showing more than 30% binding were considered to have significantly raised specific IgE against respective pollen antigen.
Pollen proteins of B. campestris, B. juncea, B. nigra, and B. napus proteins were resolved onto 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Resolved proteins were electrophoretically transferred on to nitrocellulose membrane, blocked with BSA and incubated with individual patients' sera (1:5 v/v) positive to pollen of Brassica extract. As a control, a strip blotted with respective protein extract was incubated with pooled sera of 5 healthy vounteers's (1:5 v/v). After washing, horseradish peroxidase labeled antihuman IgE (1:1000 v/v) was added and further developed [17]
The mean age of the 159 patients was 28.5 years (2-65 years) with 80:79 female vs. male ratio as in Table 1. The ratio of smokers to nonsmokers was 47:112. Of the symptomatic group, 77 patients (48.4%) suffered from BA with AR, 38 (23.9%) showed only AR, 44 had BA (27.7%) alone. The mean age of the AR patients 25.45 years, mean age of BA was 22.20 years, mean age of BA + AR is 26.91 years. The mean age of 50 healthy volunteers was 25.63 years (22-35 years) with 24:26 female and male ratio.
The protein content varied from 0.1 mg/mL (1:20 w/v) (B. juncea var. Kranti) to 1.2 mg/mL (B. napus var. Early napus) however B. campestris var. Rajendra sarson and B. nigra var. Tall recorded 1.0 mg/mL and 0.9 mg/mL of protein content. Since protein is recognized as the allergenic substance, we analyzed the protein profile of different species of Brassica pollen as reported earlier [18]. The SDS-PAGE protein profile of four Brassica pollen species is provided in Fig. 1. The extracts fractionated in to 14-17 bands in the molecular weight (mol wt) range of 14-110 kilo Daltan (kDa). Low mol wt protein of below 15 kDa and high mol wt protein of 110 kDa were separated in all the four species.
Among 4 species of Brassica pollen, B. campestris showed highest (2+ and above) skin prick response in 13.2% cases tested, followed by B. juncea with 11.9% and B. nigra showing positivity in 5.0% cases. The lowest skin reactivity was recorded with B. napus pollen in 4.4% cases only. When the mild positive skin reaction of 1+ was also included, 23.9% showed positivity to B. campestris, 26% to B. juncea, 16.9% to B. nigra and 12.5% to B. napus pollen extract (Table 2).
Out of the 30 subjects showing skin positivity (2+ and above) to at least one species of Brassica pollen, 17 (56.6%) cases reacted to at least 2 species of Brassica pollen or more. A total of 12 cases showed positivity to B. campestris and B. juncea simultaneously. However, if the clinically mild positive skin test positive reaction (1+) is also included, then out of 70 cases, 46 (65.7%) showed positivity to 2 or more species of Brassica pollen.
The skin test results with other common inhalant allergens on the above participants showed 22.6% positivity (2+ and above) from as high as in 27.6% cases to Cyanodon followed by 24.11% to Amaranthus and 21.4% to dust mite and least to 7.09% to Morus extract.
The total serum IgE values in the patient group as compared with control group (i.e., healthy volunteers) is significantly high (p = 0.0008) (Fig. 2A).
Total IgE in atopic patients tested varied from 12 IU/mL to 2,208 IU/mL with a mean of 200.84 IU/mL. The mean total IgE of female vs. male is 197.76 IU/mL vs.204 IU/mL. A positive correlation was observed between total serum IgE and skin test reactivity against different species of Brassica pollen (r = 0.2716) (Fig. 2B).
The mean optical density value in cases with raised specific IgE to pollen are 0.28 + 0.08 to B. campestris, 0.32 ± 0.11 to B. juncea, 0.33 ± 0.1 to B. nigra, and 0.34 ± 0.15 to B. napus pollen. While analyzing the percentage binding in the positive cases tested (1+ and above) as compared to healthy controls, 17 cases showed >30% binding to B. campestris, 14 against B. juncea, 11 cases against B. nigra, and 10 cases against B. napus as in Table 3. A positive correlation as in Fig. 2C was observed between the specific IgE titers in different groups of patients with intensity of skin positivity (r = 0.70) against pollen of different species of Brassica.
The IgE binding proteins in different Brassica extracts as identified by immunoblot is presented. Pooled sera of ELISA positive patients against B. campestris pollen antigen showed 10 IgE binding protein fractions as in Fig. 3. They had mol wt of 15, 20, 25, 32, 37, 47, 56, 76, 87, and 90 kDa. In 17 cases positive to B. campestris, protein fraction of mol wt 20, 25, 32, 37, 56, 76, 87, and 90 kDa were detected as IgE binding in more than 70% of cases (Table 4).
Sera of ELISA positive patients against B. juncea pollen antigen showed 10 IgE binding protein fractions as in Fig. 4. They had mol wt of 15, 20, 25, 32, 37, 47, 56, 76, 87, and 90 kDa. Protein fraction of mol wt 56, 47, and 37 kDa are of high intensity. Protein fraction of mol wt 15, 20, 25, 32, 37, 56, and 90 kDa were detected as IgE binding in more than 70% of cases (Table 4)
Sera of ELISA positive patients against B. nigra pollen antigen showed 7 IgE binding protein fractions as in Fig. 5. They had mol wt of 15, 47, 56, 76, 87, and 90 kDa, protein fraction of 67 kDa was also detected as IgE binding. Protein fractions of 15, 56, 67, 76, 87, and 90 kDa were detected in more than 70% of cases (Table 4)
Sera of ELISA positive patients against B. napus pollen antigen showed 8 IgE binding protein fractions as in Fig. 6. In B. napus, protein fraction of 32, 37, 47, 56, 67, 76, 87, and 90 kDa were identified as IgE binding. Protein fraction of mol wt 87, 76, and 67 kDa are of high intensity. Protein fraction of mol wt 56, 76, 87, and 90 kDa was detected in more than 70% of cases (Table 4).
The IgE binding protein fractions identified in different species of Brassica pollen is analyzed in Table 5. Protein fraction of 90, 87, 76, 56, 47 kDa were recognized by all the 4 species. B. campestris and B. juncea showed similar IgE binding protein fractions. Protein fraction of 67 kDa is recognized as IgE binding in B. nigra and B. napus only (Fig. 6).
Allergic diseases such as BA, AR, and atopic dermatitis are dramatically increasing all over the world including developing countries like India. Skin reactivity in respiratory allergic patients against Brassica pollen suggests it as an important aeroallergen [19]. According to Food and Agricultural Organization (FAO) Statistical data 2004, India ranks third in rapeseed-mustard production, lead by China and Canada respectively. However, precise scientific information on allergenicity to B. campestris, B. juncea, B. nigra, and B. napus being commonly cultivated in India has been lacking. With the increase in the acreage of the crop, concern has grown with regard to human health particularly to allergens. We studied the IgE mediated sensitization pattern as well as heterogeneity in allergenicity to the four cultivated species of Brassica in patients of respiratory allergy from India.
Allergological data about OSR is scarce and controversial. In our study, we have analyzed the sensitization pattern to the four species of Brassica pollen on the same set of atopic cases using SPT, ELISA, and Immunoblot analysis. Among atopic cases, 21.38% had positive SPT to at least one of the species of Brassica. Variability in skin reactivity was observed with 13.20% positivity against B. campestris as compared to 4.4% against B. napus pollen. Similar to this, Castro et al. [20] also observed significant variations in the average reactivity of allergic patients to SPT's to pollen from different olive tree cultivars. However, lower sensitization to B. nigra and B. napus as compared to B. campestris and B. juncea might also be attributed to the fact that the latter are the most extensively cultivated species in India. Thus greater the cultivation provides high exposure of pollen to population and thus higher sensitization according to Thommen's postulate [21]. Most of the cases (65.7%) showed sensitization to at least two species of Brassica. In the majority of cases, sensitization to rape was found together with multiple sensitizations to other inhalant allergens supporting the view that rape hypersensitivity typically is an additional phenomenon in atopics [5].
The total serum IgE concentration in the patient group as compared with control groups is highly significant. The IgE levels in Indian allergic patients is significantly related to atopy, but due to wide overlap of IgE levels in patients and healthy subjects, its diagnostic significance in Indian population seems to be limited [22]. Therefore, to establish sensitization, specific IgE to Brassica species was considered as diagnostic tool for allergenicity. Raised allergen specific IgE to respective Brassica pollen has been recorded in skin test positive cases. The difference in mean specific IgE values obtained against B. campestris, B. juncea, B. nigra, and B. napus is not statistically significant.
In an earlier study, Singh et al. [23] reported only 4 protein fractions as IgE binding as compared to 10 IgE binding fractions in our study with pollen of B. campestris. This difference might be caused by the use of very different protein extraction and by the use of pollen from commercial sources, corresponding to undetermined cultivars. Interestingly, protein fractions of mol wt 47, 56, 76, 87, 90 kDa are identified as IgE binding in all the four species of Brassica and provides good source of allergen standardization. Hemmer et al. [6] also identified IgE binding protein fractions of 12/14 and in the high molecular weight range at 33, 42, 51, 58/61, and 70 kDa in B. napus. Some workers also reported allergens of 14 kDa as well as high molecular weight cluster (27-69 kDa) comprising of six cross reactive allergenic protein in oilseed rape pollen [6, 19]. Protein fractions of mol wt 15, 20, and 25 kDa in B. nigra and 20, 25, 32, and 37 kDa in B. napus are not recognized as IgE binding. Lower cultivation leading to low airborne pollen suspected to cause lower sensitization might explain this variability. Focke et al. [19] also indicated that allergen profiles seem to be similar among pollen of Brassica species as comparable allergens were described by them in B. napus and by Singh et al. [23] in B. campestris. There is a need to rule out number of protein fractions showing IgE binding to all four species for the glycoprotein suspected to be cross reactive protein [24] in some cases.
In our study, the different species of Brassica were cultivated in experimental field especially for this study. Hence monitoring on each step has checked the intermingling of pollen on species level, which would not have been possible otherwise in Indian scenario where there is no clear demarcation in the growing areas on species level of Brassica. Our study confirms that patients display different SPT responses when tested with pollen extracts of the same genus but different species. The relative amount of major allergen as well as a putative linkage between patient origin and the prevalence of allergy to local species deserve close attention. Our results further conclude that Brassica pollen does act as an allergen and hence should be considered as a potentially relevant factor in pollen allergy and OSR attributed adverse effects in particular along with other common inhalant allergens. For clinical safety and efficacy of the extracts, the present study will help in standardization of pollen extract of Brassica being commercially produced and also used for diagnosis and immunotherapy in India.
Figures and Tables
Fig. 1
Comparative protein profile of water soluble extracts of four different species of Brassica pollen. M, Marker; Lane 1, Brassica campestris var. Rajendra sarson; 2, Brassica juncea var. Kranti; 3, Brassica nigra var. Tall; 4, Brassica napus var. Early napus.
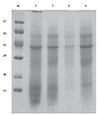
Fig. 2
(A) Comparison of total IgE with intensity of skin test reactions against pollen of Brassica in atopic cases. 1, healthy volunteer; 2, negative skin test; 3, 1+ skin test response; 4, 2+ skin test response; 5, 3+ and above skin test response. (B) Comparison of specific IgE with intensity of skin test reactions against pollen of Brassica in atopic cases. 1, negative skin test; 2, 1+ skin test response; 3, 2+ skin test response; 4, 3+ and above skin test response.

Fig. 3
Immunoblot of pollen of Brassica campestris carried out with individual sera positive to it. M, molecular weight standards; P, pooled sera of positive sera; Lane 1-17, individual sera positive to pollen of B. campestris; HV, pooled sera of healthy volunteers.

Fig. 4
Immunoblot of pollen of Brassica juncea carried out with individual sera positive to it. M, molecular weight standards; P, pooled sera of positive sera; Lane 1-14, individual sera positive to pollen of B. juncea; HV, pooled sera of healthy volunteers.

Fig. 5
Immunoblot of pollen of Brassica nigra carried out with individual sera positive to it. M, molecular weight standards; P, pooled sera of positive sera; Lane 1-11, individual sera positive to pollen of B. nigra; HV, pooled sera of healthy volunteers.
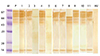
Fig. 6
Immunoblot of pollen of Brassica napus carried out with individual sera positive to it. M, molecular weight standards, P, pooled sera of positive sera; Lane 1-10, individual sera positive to pollen of B. napus; HV, pooled sera of healthy volunteers.

Table 2
Skin prick test results with pollen extracts from four different species of Brassica conducted on 159 patients with respiratory symptoms
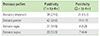
Table 3
Specific IgE (optical density) of patients showing increased percent (%) binding to different species of Brassica pollen

ACKNOWLEDGEMENTS
This work had been funded by Council of Scientific & Industrial Research-University Grant Commission. We are thankful to Raghuni Prasad for field help and healthy volunteers who participated in the study.
References
1. Colldahl H. Rape pollen allergy; report of a case. Acta Allergol. 1954; 7:367–369.
2. Gaur RD. Aeropalynology of Meerut I: pollen grains. J Indian Bot Soc. 1978; 57:353–365.
3. Singh AB, Babu CR. Survey of atmospheric pollen allergens in Delhi: seasonal periodicity. Ann Allergy. 1982; 48:115–122.
4. Singh AB. Pollen production in common allergenic plants of Delhi and their atmospheric prevalence. Sci Cult. 1984; 50:65–67.
5. Fell PJ, Soulsby S, Blight MM, Brostoff J. Oilseed rape: a new allergen? Clin Exp Allergy. 1992; 22:501–505.
6. Hemmer W, Focke M, Wantke F, Jager S, Gotz M, Jarisch R. Oilseed rape pollen is a potentially relevant allergen. Clin Exp Allergy. 1997; 27:156–161.

7. Hemmer W. The health effects of oilseed rape: myth or reality? No clear evidence that it has adverse effects on health. BMJ. 1998; 316:1327–1328.
9. Murphy DJ. Is rapeseed really an allergenic plant? Popular myths versus scientific realities. Immunol Today. 1999; 20:511–514.

10. Galloway D. Oilseed rape: allergen or irritant? Clin Exp Allergy. 2000; 30:308–309.
11. Bugur I, Arner B. Rape pollen allergy. Scand J Respir Dis. 1978; 59:222–227.
12. Singh RP, Kumar A. New horizons for Brassica carinata under resource constraints in northern plains of India. In : Proceedings of the 10th International Rapeseed congress; 1999 Sep 26-29; Canberra, Australia. 1999. p. 463–467.
13. Cour P, Loublier Y. Sampling and preparation of pollen for microanalysis. Rev Franc Allergol. 1980; 20:197–199.
14. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.

15. Singh AB, Malik P, Parkash D, Gupta CK. Biological standardization of pollen allergens from India. Asian Pac J Allergy Immunol. 1992; 10:103–109.
16. Sepulveda R, Longbottom JL, Pepys J. Enzyme linked immunosorbent assay (ELISA) for IgG and IgE antibodies to protein and polysaccharide antigens of Aspergillus fumigatus. Clin Allergy. 1979; 9:359–371.

17. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979; 76:4350–4354.

18. Shahi S, Katiyar RK, Bhatnagar AK, Singh AB. Soluble and nonsoluble protein assay for antigenic extracts from pollen and seeds of mustard (Brassica spp.). Allergy Asthma Proc. 2008; 29:78–87.

19. Focke M, Hemmer W, Hayek B, Gotz M, Jarisch R. Identification of allergens in oilseed rape (Brassica napus) pollen. Int Arch Allergy Immunol. 1998; 117:105–112.

20. Castro AJ, de Dios Alche J, Cuevas J, Romero PJ, Alche V, Rodriguez-Garcia MI. Pollen from different olive tree cultivars contains varying amounts of the major allergen Ole e 1. Int Arch Allergy Immunol. 2003; 131:164–173.

21. Coca AF, Thommen AA, Walzer M. Asthma and hay fever in theory and practice. Springfield: Thomas;1931.
22. Sharma S, Kathuria PC, Gupta CK, Nordling K, Ghosh B, Singh AB. Total serum immunoglobulin E levels in a case-control study in asthmatic/allergic patients, their family members, and healthy subjects from India. Clin Exp Allergy. 2006; 36:1019–1027.

23. Singh BP, Verma J, Rai D, Sridhara S, Gaur SN, Gangal SV. Immunobiochemical characterization of Brassica campestris pollen allergen. Int Arch Allergy Immunol. 1995; 108:43–48.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download