Abstract
Background
We reported that level of lipopolysaccharide (LPS) exposure determined the type of airway inflammation in a murine model of asthma.
Objective
The purpose of this study is to evaluated the role of IL-13 in low dose LPS induced murine model of asthma using IL-13 and signal transducer and activator of transcription 6 (STAT6) deficient mice.
Methods
Mice were sensitized with an intranasal application of LPS-depleted ovalbumin (OA) and different doses of LPS (0.1 and 10 µg), and then challenged intranasally with OA alone. The phenotype changes between wild type (WT) and IL-13-/- mice and between WT and STAT6-/- mice were evaluated.
Results
We confirmed again that low and high dose LPS resulted in different phenotypes of murine asthma. In the present study, we observed that phenotypes of murine asthma induced by low dose LPS were abolished in the homozygous null mutation of the IL-13 and STAT6 gene. However, those changes were not shown in mice sensitized OA plus high dose LPS.
Asthma is a chronic inflammatory lung disease associated with intermittent airflow obstruction, airway hyperresponsiveness (AHR) and the infiltration of respiratory mucosa by inflammatory cells, mainly eosinophils [1]. Multiple factors are involved in the pathogenesis of asthma, but the initiation of aberrant immune responsiveness against inhaled environmental respiratory allergens, characterized by the development of allergen-specific IgE, and the presence of allergen-specific CD4+ T cells producing IL-4, IL-5, and IL-13, but not IFN-γ, is undoubtedly a critical element [2]. However, inhaled allergens are ubiquitous, so it remains unclear why some individuals develop adaptive immune responses to allergens while others do not. In this context, lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria contaminated in household dust allergens including house dust mites [3], is of particular interest.
The priming of naive T cells to Th2 cells to produce protein antigen requires the activation of the innate immune system provoked by LPS [4-6]. Accordingly, we reported that a murine model of asthma could be developed by sensitization with allergen plus LPS and level of LPS exposure determined the type of airway inflammation, i.e., low dose LPS (0.1 µg per application) resulted in eosinophilic inflammation, whereas high dose LPS (10 µg per application) resulted in non-eosinophilic inflammation [7].
IL-13, a pleiotropic cytokine secreted from activated Th2 cells, is involved in the regulation of IgE synthesis, mucus hypersecretion, subepithelial fibrosis and eosinophil infiltration, and thus has been thought to be a key mediator in asthma pathogenesis [8]. The effect of IL-13 is mainly mediated by the signal transducer and activator of transcription 6 (STAT-6) signaling pathway [9] and partially overlaps with that of IL-4 [10]. This redundancy may be explained by a shared component of their receptors (IL-4Rα) [11]. In our previous report, we observed that low dose LPS induced phenotypes of murine asthma were affected in the absence of IL-4 but phenotypes induced by high dose LPS were not [7]. So far, the role of IL-13 in the development of low dose LPS induced phenotypes of murine asthma has been totally unknown. The purpose of this study is to evaluated the role of IL-13 in the development of phenotypes of murine asthma induced by low (0.1 µg) or high dose (10 µg) LPS using IL-13 and STAT6 deficient mice.
IL-13 deficient (IL-13-/-) and STAT6 deficient (STAT6-/-), and wild-type (WT) control mice on a C57BL/6 background were purchased from Jackson Laboratory (USA). The mice were maintained in semi-specific pathogen-free conditions. Six to eight week-old mice were used in all experiment with four mice per group. Mice were sensitized intranasally (IN) four times with 75 µg of ovalbumin (OA) on days 0, 1, 2, and 7 with or without different doses of LPS (from E. coli O55: B5; Calbiochem, USA), and then challenged two times IN with 50 µg of OA on days 14, and 15 (Fig. 1). In this experiment, low and high doses of LPS were defined as 0.1 and 10 µg, respectively, which are levels of contamination consistent with house dust allergens [7]. To evaluate asthma phenotypes, lung inflammation and methacholine AHR were evalu ated on day 16 and 17. LPS-depleted OA by endotoxin removing gel kit (Pierce, USA) was used in all experiments. Animal study protocols were approved by the Institutional Animal Care and Use Committee of Seoul National University, Korea.
Pulmonary function testing in mice was assessed using conscious, unrestrained mice by noninvasive whole body plethysmography (Allmedicus, Korea). Mice were placed in a plethysmograph chamber and exposed to an aerosol of phosphate buffer solution (PBS, basal readings) and then to methacholine at 6.25, 12.5, 25, and 50 mg/mL. Aerosols were generated using an ultrasonic nebulizer and drawn through the chamber for 3 min. Enhanced pause (Penh) readings were taken for 3 min and averaged. We previously confirmed a direct correlation between Penh and airway resistance in response to methacholine challenge [7].
Lung and bronchoalveolar lavage (BAL) samples were obtained as previously described [7]. Briefly, mice were anesthetized and their trachea was isolated by blunt dissection. A small caliber tube was inserted and secured in the airway. Two successive volumes of 0.75 µL of PBS were instilled and gently aspirated and these two volumes were pooled. Each BAL sample was centrifuged and the supernatants were stored at -70℃ until use. The total numbers of inflammatory cells were counted after dilution of cell pellets with 50 mL of PBS. After Diff-Quick staining (Dade Behring, USA) of BAL pellets in a cytospin preparation, types of inflammatory cells were determined by counting 300 cells, which were classified as macrophages, lymphocytes, neutrophils, or eosinophils. OA-specific IgE levels in serum samples obtained during animal experiments were determined as previously described [7].
Significant differences among groups were assessed using the Student's t test, ANOVA, or a Wilcoxon rank sum test. For multiple comparisons, ANOVA was initially used and if significant differences were found individual two-tailed unpaired t or Wilcoxon rank sum tests between pairs of groups were used.
We confirmed that our protocol reproduced what we reported before [7]. Namely, methacholine AHR following allergen (OA) challenge was more enhanced in mice sensitized with OA plus LPS, independent of LPS dose, than in those sensitized with OA or PBS alone. BAL eosinophil counts were higher in mice sensitized with OA plus low dose LPS (0.1 µg) than in those sensitized with OA plus high dose LPS (10 µg), whereas BAL neutrophil counts were greater in mice sensitized with OA plus high dose LPS than in mice sensitized with OA plus low dose LPS.
BAL cellularity was significantly lower in IL-13-/- mice sensitized with OA plus low-dose LPS compared to WT mice sensitized in the same manner, but similar in these mice when sensitized with OA plus high dose LPS (Fig. 2A). Moreover, methacholine AHR was not observed in IL-13-/- mice sensitized with OA plus low dose LPS, but was observed in IL-13-/- mice sensitized with OA plus high dose LPS as prominent as WT mice sensitized in the same manner (Fig. 2B). In terms of immunologic aspect, enhanced production of OA-specific IgE in WT mice sensitized with OA plus low dose LPS was significantly attenuated in IL-13-/- mice, whereas IL-13-/- mice sensitized with OA plus high dose LPS showed as similar levels of OA-specific IgE as WT mice sensitized in the same manner (Fig. 2C).
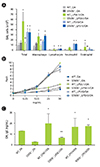
Lung inflammation enhanced by low dose LPS was found to be markedly reduced in STAT6-/- mice (Fig. 3A). Moreover, methacholine AHR was significantly inhibited in STAT6-/- mice sensitized with OA plus low dose LPS compared to WT mice sensitized in the same manner (Fig. 3B). However, lung inflammation and methacholine AHR induced by OA plus high dose LPS showed no significant changes between STAT6-/- and WT mice (Figs. 3A and B). The same trend was observed for OA-specific IgE (Fig. 3C).
In the present study, we confirmed again that low and high dose LPS resulted in different phenotypes of murine asthma. We observed that phenotypes of murine asthma induced by low dose LPS were abolished in the homozygous null mutation of the IL-13 and STAT6 gene. However, those changes were not shown in mice sensitized OA plus high dose LPS. Considering our previous finding that low dose LPS induced phenotypes of murine asthma were depend on the IL-4 signaling [7], phenotypes of murine asthma induced by low dose LPS were mediated totally by Th2 type immune mechanisms.
Murine models of asthma have been found to be extremely useful for examinations of the basic mechanisms of allergic inflammation and immunologic response underlying human bronchial asthma [12]. In the majority of current asthma models, mice are sensitized by intraperitoneal (IP) allergen injection, often together with a Th2 skewing adjuvant, such as alum [13]. However, allergen exposure by the IP route is not physiologic, at least, in the case of bronchial asthma. On the other hand, the administration of substances to mice by the IN route is believed to more effectively deliver allergens [14, 15]. It was demonstrated that IN instillations successfully delivered allergens to the lower respiratory tract, although this was found to be heavily influenced by delivered volume and level of anesthesia [16]. Nevertheless, IP sensitization is still commonly used, which may be because of the lack of a validated IN applied immune modulator. As mentioned before, LPS is ubiquitous in the environment and is often present in high concentrations in organic dusts [17], in air pollution [18], and in household dusts [19]. Emerging evidence from murine models of asthma indicates that, like Th1 immunity, the priming of naive T cells to Th2 cells by protein antigens also requires the activation of the innate immune system provoked by LPS [4-6]. The results of the present study also support this mechanism. In the present study, low dose LPS (0.1 µg) provoked Th2 immune responses. Mean airborne levels ranging from 0.01 to 30 ng/m3 have been measured in in urban homes [3, 20]. Assuming a mean ventilation rate of 10 L/min, these levels correspond to a daily cumulative LPS exposure of 0.14-432 ng (0.00014-0.432 µg) in urban homes. Moreover, it has been reported that mice are 2 to 3 log less sensitive to LPS than humans [21]. Thus it is probable that an exposure to 0.1 µg of LPS in mice mimics a natural exposure to LPS in human in a daily urban life. Taken together, we may say that IN sensitization with allergen plus low dose LPS provides a more physiologic, and therefore, acceptable murine model of asthma of asthma.
IL-13 is thought to be a central mediator of asthma and required for the development of experimental asthma independently of IL-4 [22]. Recently, several promising therapies for asthma targeting the IL-13/IL-4/STAT6 pathway are in development [23]. Moreover, those therapies have demonstrated effectiveness in human and murine model of asthma [24-27]. However, the role of IL-13 in innate immunity in human allergic asthma has been still unknown. In the present study using a more physiologic model of murine asthma, we demonstrated that immunomodulating effects of LPS on allergen induced responses are dependent on IL-13 signaling. Although further mechanistic studies should be followed, we are sure that our results provided a new insight in understanding of the potential role of IL-13 in innate immunity in human allergic asthma.
Figures and Tables
Fig. 1
Protocol for a murine model of asthma. AHR, airway hyperresponsiveness; Def, gene deficient mice; IN, intranasally; LPS, lipopolysaccharide; OA, ovalbumin; WT, wild type mice.

Fig. 2
Lung inflammation, airway hyperresponsiveness and serum OA-specific IgE in IL-13 deficient mice. (A) Lung inflammation. *p < 0.05 vs. WT_OA and IL-13-/-_OA groups; **p < 0.05 vs. WT_LPS0.1/OA groups. (B) Methacholine airway hyperesponsiveness. (C) Serum OA-specific IgE. *p < 0.05 vs. WT_OA and IL-13-/-_OA groups; **p < 0.05 vs. WT_LPS0.1/OA groups. BAL, bronchoalveolar lavage; OA, ovalbumin; Penh, enhanced pause; WT, wild type. One experiment representative of three is shown.
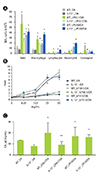
Fig. 3
Lung inflammation, AHR and serum OA-specific IgE in STAT6 deficient mice. (A) Lung inflammation. *p < 0.05 vs. WT_OA and STAT6-/-_OA groups; **p < 0.05 vs. WT_LPS0.1/OA groups. (B) Methacholine airway hyperesponsiveness. (C) Serum OA-specific IgE. *p < 0.05 vs. WT_OA and STAT6-/-_OA groups; **p < 0.05 vs. WT_LPS0.1/OA groups. STAT6, signal transducer and activator of transcription 6; BAL, bronchoalveolar lavage; OA, ovalbumin; Penh, enhanced pause; WT, wild type. One experiment representative of three is shown.
ACKNOWLEDGEMENTS
This work was supported financially by a grant from Seoul National University Hospital (#0420060080).
References
1. Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994; 12:295–335.

3. Park JH, Spiegelman DL, Burge HA, Gold DR, Chew GL, Milton DK. Longitudinal study of dust and airborne endotoxin in the home. Environ Health Perspect. 2000; 108:1023–1028.

4. Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002; 168:4524–4530.

5. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002; 196:1645–1651.

6. Wan GH, Li CS, Lin RH. Airborne endotoxin exposure and the development of airway antigen-specific allergic responses. Clin Exp Allergy. 2000; 30:426–432.

7. Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007; 178:5375–5382.

8. Brightling CE, Saha S, Hollins F. Interleukin-13: prospects for new treatments. Clin Exp Allergy. 2010; 40:42–49.

9. Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol. 2000; 105:1063–1070.

10. Zurawski G, de Vries JE. Interleukin 13 elicits a subset of the activities of its close relative interleukin 4. Stem Cells. 1994; 12:169–174.

11. Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, Leonard WJ. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995; 2:331–339.

12. Kumar RK, Foster PS. Modeling allergic asthma in mice: pitfalls and opportunities. Am J Respir Cell Mol Biol. 2002; 27:267–272.
13. Kips JC, Anderson GP, Fredberg JJ, Herz U, Inman MD, Jordana M, Kemeny DM, Lötvall J, Pauwels RA, Plopper CG, Schmidt D, Sterk PJ, Van Oosterhout AJ, Vargaftig BB, Chung KF. Murine models of asthma. Eur Respir J. 2003; 22:374–382.

14. Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997; 155:661–669.

15. Chapoval SP, Nabozny GH, Marietta EV, Raymond EL, Krco CJ, Andrews AG, David CS. Short ragweed allergen induces eosinophilic lung disease in HLA-DQ transgenic mice. J Clin Invest. 1999; 103:1707–1717.

16. Southam DS, Dolovich M, O'Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002; 282:L833–L839.

17. Rylander R, Haglind P, Lundholm M. Endotoxin in cotton dust and respiratory function decrement among cotton workers in an experimental cardroom. Am Rev Respir Dis. 1985; 131:209–213.
18. Bonner JC, Rice AB, Lindroos PM, O'Brien PO, Dreher KL, Rosas I, Alfaro-Moreno E, Osornio-Vargas AR. Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from Mexico City. Am J Respir Cell Mol Biol. 1998; 19:672–680.

19. Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996; 154:1641–1646.

20. Olenchock SA, May JJ, Pratt DS, Morey PR. Occupational exposures to airborne endotoxins in agriculture. Prog Clin Biol Res. 1987; 231:475–487.
21. Berczi I, Bertók L, Bereznai T. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Can J Microbiol. 1966; 12:1070–1071.

22. Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998; 282:2261–2263.

23. Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012; 130:829–842.

24. Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.

25. Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, She D, Kell C, May RD, Geba GP, Molfino NA. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013; 41:330–338.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download