Abstract
Background
The main symptoms of allergic rhinitis (AR) are sneezing, rhinorrhea and nasal obstruction. It was reported that the nasal skin temperature after intranasal administration of histamine or grass pollen rose. In patients with AR, the levels of nerve growth factor (NGF) and vascular endothelial growth factor (VEGF) have increased in nasal fluids and mucosa.
Objective
The present study were to determine the temperature changes of the nose in rat allergic rhinitis model, and if olopatadine, an antiallergic agent with histamine H1 receptor antagonistic action, proved to be effective, were studied the productions of NGF and VEGF in nasal lavage fluids (NALF). In the present study, we used ovalbumin (OVA)-sensitized rats as an animal model of nasal allergy and examined the effects of olopatadine on the skin temperature of the nose area, and the productions of NGF and VEGF in NALF.
Methods
The temperature changes of the nose area were carried out with thermo tracer in rat passively sensitized with OVA antiserum. The numbers of sneezing episodes were counted and, NGF and VEGF levels in NALF were examined using the specific ELISA.
Results
In OVA-sensitized rats, the number of sneezing episodes increase and the nasal skin temperature rise were provoked after OVA challenge. The levels of NGF and VEGF in NALF also were increased. Olopatadine reduced the increased frequency of sneezing and the nasal skin temperature rise. It also inhibited the increased NGF and VEGF productions in NALF.
Conclusion
The nasal skin temperature after OVA challenge rose even in OVA-sensitized rats. These results suggest that the suppression of the increased NGF and VEGF levels might partially be involved in the improvement of allergy-like behavior (sneezing and nasal skin temperature rise) by the treatment of olopatadine.
Histamine is one of the most important mediators in allergy. In clinical study, wheal and flare tests by histamine iontophoresis stimulations have been intensively demonstrated and, have been used to compare antihistaminic effects at the skin level [1, 2]. At the nose level, antihistaminic effects have been compared with nasal flow by rhinomanometry, nasal secretion and nasal symptom score (including: rhinorrhea, nasal itching, sneezing, and nasal obstruction) by histamine or allergen challenge [3, 4].
Intranasal administration of histamine or grass pollen were induced a dose-dependent rise in nasal skin temperature [5]. The effects of antihistamines using facial thermography were consistent with the results of nasal symptom score and wheal and flare. It was reported that facial thermography is an objective, noninvasive and sensitive method to study antihistaminic activity at the nose level [6].
Symptoms of allergic rhinitis (AR) are sneezing, nasal rubbing, nasal congestion and rhinorrhea, caused by the interaction between chemical mediators and sensory nerves through activation of specific receptors. In the clinical study, nerve growth factor (NGF) and neuropeptides, such as substance P, neurokinin A, and the calcitonin gene-related peptide, are up-regulated and their release in nasal fluids has been demonstrated after allergen or hypertonic saline exposure [7-9]. Furthermore, nasal vasodilatation and increased vascular permeability are important features of AR. Angiogenic factor vascular endothelial growth factor (VEGF) has also been shown to increase in the nasal mucosa of patients with AR, as a result of the increase in nasal vascular permeability and congestion [10]. VEGF participates in nasal mucosa swelling from increased blood vessel permeability in an immediate reaction of AR [11]. VEGF may therefore play an important role in nasal mucosal inflammation in AR.
Olopatadine hydrochloride tablet (Olopatadine; Kyowa Hakko Kirin, Japan) is an antiallergic agent with histamine H1 receptor antagonistic action, prescribed for patients with signs and symptoms of AR, chronic urticaria, eczema dermatitis, prurigo, pruritis cutaneous, psoriasis vulgaris and erythema exsudativum multiform [12]. Olopatadine ophthalmic solution, which inhibits the proinflammatory activity of conjunctival mast cells, is an effective therapy for allergic conjunctivitis [13]. Additionally, olopatadine nasal spray is a steroid-free prescription nasal spray that works quickly to fight the symptoms of seasonal AR [14].
We previously reported that olopatadine reduced nasal allergy signs in toluene-2,4-diisocyanate-challenged rats and inhibited the increased NGF and VEGF levels in nasal lavage fluids (NALF) [15].
The purposes of the present study were, first, to determine the temperature changes of the nose, in an animal model of nasal allergy induced by intranasal challenge with ovalbumin (OVA), and, second, if olopatadine proved to be effective, to investigate the productions of NGF and VEGF in NALF.
Male 6-week-old Wistar rats were purchased from Charles River Japan (Japan). Animals were maintained at 19-25℃ with 30-70% of humidity on a 12 h light/dark cycle (light on at 7:00), and had free access to commercial pellets and water. Experiments were performed in accordance with recommendations of the institutional animal care and use committee of the Kyowa Hakko Kirin Co., Ltd.
Drugs and reagents used in the study were olopatadine hydrochloride (Olopatadine; Kyowa Hakko Kirin, Japan), prednisolone (Sigma-Aldrich, USA), albumin (OVA; Sigma-Aldrich, USA) and Evans blue (Sigma-Aldrich, USA). Olopatadine was dissolved in distilled water. Prednisolone was suspended in 0.5 w/v% methylcellulose solution.
OVA was prepared at 400 µg/mL in saline and precipitated at a 1:1 ratio with Al(OH)3 (20 mg/mL). Rats were immunized by an intraperitoneal injection of 200 µg OVA (1 mL OVA-Al(OH)3 suspension) at Days 1, 2, 3, and 11, following the sensitization procedure of Shimizu et al. [16] (Fig. 1). Heat-killed Bordetella pertussis bacilli (1010 in 0.6 mL saline) were given by a foot pat injection on Day 1 as an adjuvant.
At Days 20 or 21, 25 µL of saline containing 2.5 mg of OVA was instilled into both airways of nasal cavity for 2 or 4 consecutive days. Sham-challenged rats received 25 µL saline in the same manner. Olopatadine (3 and 10 mg/kg/day) and prednisolone (10 mg/kg/day) were orally administered at a volume of 1 mL/100 g body weight before OVA challenge.
Temperature and relative humidity were continuously monitored and maintained constant at 19-25℃ and 30-70% of humidity in the room. At Days 21 or 22, the skin temperature of the nose area (square centered on animal's nose) was carried out with thermo tracer (TH5100 GP-IB; NEC, Japan) at before and 15 min to 6 h after the OVA challenge. The changes in skin temperature were showed the difference from the temperatures at before the OVA challenge by the average and maximum temperature.
Rats were placed into an observation cage divided into compartments (14 × 20 × 15 cm) for 1 h of habituation. After intranasal challenge with OVA, number of sneezing episodes, a sign of nasal allergy, was counted in a blinded manner during a 10-min period just after exposure.
At 6 h after the final intranasal challenge, rats were anesthetized with sodium pentobarbital. Nasal lavages were done using catheter. The catheter with rubber tubing covering the head of tube was inserted into the tracheal opening the direction of upper airway. Both sides of the nasal cavity were lavaged with PBS containing a protease inhibitor and centrifuged. Each supernatant was aliquoted and frozen at -35℃. ELISA for NGF (Promega, USA) and VEGF (R&D Systems, USA) were conducted according to the manufacturer's instructions. The optical density of each well was determined using a microplate reader (THERMOmax™; Molecular Devices, USA).
Data were presented as means ± SE. The Aspin-Welch test or Student's t-test following the F-test, was used for analysis of differences between two groups. Multiple comparisons among treatment groups were assessed by one-way analysis of variance, followed by the Dunnett's test or the Steel test. Values of p < 0.05 were considered statistically significant.
Skin temperature of the nose area after intranasal challenge with OVA was risen (Fig. 2). As shown in Fig. 3A, skin maximum temperature of the nose area at 0.25, 2 and 6 h after intranasal challenge with OVA was significantly risen compared with saline-treated animals (p = 0.0324, p = 0.0095, p = 0.0031), respectively. Skin mean temperature at 0.25, 0.5, 1, 2, 3 or 6 h after OVA challenge was significantly risen compared with saline-treated animals (p = 0.0007, p = 0.0044, p = 0.0406, p = 0.0018, p = 0.0179, p = 0.0161), respectively (Fig. 3B). Skin minimum temperature of the nose area did not change with olopatadine-treated animals (data not shown). Olopatadine at 10 mg/kg/day significantly suppressed the risen maximum temperature at 0.25, 2, 4 and 6 h (p = 0.0268, p = 0.0349, p = 0.0137, p = 0.0028), respectively. On the mean temperature, olopatadine significantly suppressed the risen maximum temperature at 2 or 6 h (p = 0.0287, p = 0.0128), respectively.
Prednisolone at 10 mg/kg/day significantly suppressed the risen maximum temperature at 4 and 6 h (p = 0.0284, p = 0.0008) and the risen mean temperature at 6 h (p = 0.0011), respectively.
In OVA-sensitized rats challenged with OVA, there was an increase in the number of sneezing episodes compared to saline-challenged animals (p = 0.0002) (Fig. 4). Olopatadine (3 and 10 mg/kg/day) significantly suppressed the increased frequency of sneezing by 49.3% and 85.0% (p = 0.0016, p < 0.0001), respectively. Prednisolone also significantly suppressed the increased number of sneezing by 87.1% (p < 0.0001).
As shown in Fig. 5A, level of NGF in the NALF at 6 h after OVA challenge was significantly increased compared to saline-treated animals (p = 0.0175, p = 0.0003), respectively. Olopatadine at 10 mg/kg/day significantly inhibited the increased levels of NGF by 85.4% (p = 0.0282), respectively. Prednisolone significantly inhibited the increased levels of NGF by 80.9% (p = 0.0420).
As shown in Fig. 5B, level of VEGF in the NALF at 6 h after OVA challenge was significantly increased compared to saline-treated animals (p = 0.0246). Olopatadine at 10 mg/kg/day significantly inhibited the increased levels of VEGF by 98.5% (p = 0.0193). Prednisolone significantly inhibited the increased levels of VEGF by 87.4% (p = 0.0301).
In OVA-sensitized rats, the number of sneezing episodes increases and the nasal skin temperature rises were provoked after OVA challenge. Olopatadine reduced the increased frequency of sneezing and the nasal temperature rise. The levels of NGF and VEGF in NALF also were increased by OVA challenge. Olopatadine inhibited the increased NGF and VEGF production.
The efficacy of antihistamines on rhinitis was compared by measuring nasal symptoms and rhinomanometry, which olopatadine is considered effective for symptoms such as sneezing, rhinorrhea and nasal obstruction [3, 17-19]. In clinical study, the antihistaminic activity of levocetirizine and fexofenadine was assessed using facial thermography during nasal provocation tests with histamine and allergen [6]. In this study, olopatadine reduced the sneezing and the risen nasal temperature in the OVA-induced allergic rhinitis model. Therefore the assay using thermography also could become a useful clinical indicator in animal model.
In this study, skin minimum temperature of the nose area did not changed, but skin maximum and mean temperature rise were observed during 0.25-3 h, and those rises were observed again at 6 h after OVA challenge. The main symptoms of AR are observed in the early phase and the late phase of the disease. The early phase response, characterized by sneezing, rhinorrhea and nasal congestion, occurs within a few minutes of antigen exposure and subsides after 30-90 min. In contrast, the late phase reaction, characterized mainly by nasal congestion, begins around 4-8 h after the early phase response [20]. Olopatadine significantly inhibited the decrease in the nasal cavity volume at the early phase and the late phase after the antigen challenge [21].
Nasal congestion may be due to the increased nasal blood flow and nasal temperature increase may be involved in blood flow; blood flow is thought to cause the swelling of nasal mucosa. The swelling of nasal mucosa by antigen are considered a direct effect on the nasal mucosa vasculature of chemical mediators such as histamine, cysteinyl leukotrienes and PAF [22-24]. It is also reported that capsaicin sensitive sensory nerve reflexes were related to nasal blockage [25]. It has been shown that olopatadine inhibits the release and the action of histamine, the release of peptide leukotrienes [26], the production of PAF and leukotriene B4, and the release of neuropeptides [27]. Accordingly, the efficacy of olopatadine on nasal temperature increase is likely to be based on these actions; that is, inhibitory actions on the release of histamine, peptide leukotrienes and neuropeptides, and on the production of PAF as well as based on the antagonistic actions on histamine.
VEGF is produced from mast cell and is involved in type I allergy [28]. Therefore, VEGF is thought to contribute to the swelling of nasal mucosa by the increased vascular permeability in the early phase of AR. It is suggested that olopatadine is suppressing the swelling of nasal mucosa by the inhibitory effect of LTs [21]. Relationship between blood flow, swelling of nasal mucosa and nasal temperature in this model is not clear. The inhibitory effect on nasal temperature of olopatadine was suggested that it is involved H1 antagonistic effects and the inhibition of LTs release in addition to the inhibition of increased histamine and VEGF.
NGF increases the sensitivity to sneeze reflex and makes an increase in nasal rhinorrhea and plasma leakage by sensory nerve stimulation [29]. The amount of NGF in NALF of patients with AR is increasing compared to healthy volunteers, it were additional increased by the antigenic exposure [30]. Neuropeptides positive nerve fibers are increased in nasal mucosa of patients with AR. Neuropeptides may exacerbate further exposure to antigen reaction by encouraging the extension of nerve fibers. In this study, olopatadine inhibited the NGF production. These results suggest that the suppression of the increase in NGF might partially be involved in the improvement of allergy-like behavior and nasal temperature increase.
Olopatadine reduced the risen nasal temperature in the OVA-induced allergic rhinitis model. Therefore the assay using thermography could become a useful clinical indicator in animal model. More detailed studies will be required to ascertain the precise mechanism of action of olopatadine on nasal blockage. These results suggest that the suppression of the increased NGF and VEGF levels might partially be involved in the improvement of allergy-like behavior (sneezing and nasal skin temperature rise) by the treatment of olopatadine.
Figures and Tables
Fig. 1
Protocol for ovalbumin (OVA) sensitization and challenge. OVA was prepared at 400 µg/mL in physiological saline and precipitated at 1:1 ratio with Al(OH)3 (20 mg/mL). Rats were sensitized by intraperitoneal injections of 200 µg OVA (1 mL OVA-Al(OH)3 suspension) on Days 1, 2, 3 and 11. Bordetella (B.) pertussis (1010 organisms in physiological saline) were injected into the four footpads on Day 1. Rats were challenged with OVA in physiological saline (100 mg/mL, 25 µL × 2) into the bilateral nasal cavities using a micropipette for 2 or 4 consecutive days. Drugs were orally administered 1 h before each OVA challenge. NALF: nasal lavage fluids.
Fig. 2
Nasal skin temperature change after the last nasal ovalbumin (OVA) challenge. The images of thermograph were at 1 h after the OVA challenge. The temperature is color coded. A change from blue to red indicates an increase in temperature. Olopatadine was orally administered 1 h before the OVA challenge.
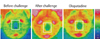
Fig. 3
Nasal skin temperature change after the last nasal ovalbumin (OVA) challenge. Maximum (A) and mean (B) temperature change. Olopatadine and prednisolone were orally administered 1 h before the OVA challenge. The nasal skin temperature was measured at 0.25, 0.5, 1, 2, 3, 4 and 6 h after the OVA challenge. □: saline, ●: OVA challenge, □: olopatadine, ■: prednisolone. Each point represents the mean ± SE of 6 rats. †p < 0.05, ††p < 0.01, †††p < 0.001: significantly different from the OVA challenged group.

Fig. 4
Effects of olopatadine and prednisolone on the number of sneezing induced by the ovalbumin (OVA) challenge. Olopatadine and prednisolone were orally administered before the OVA challenge. The number of sneezing was measured for 10 min. Each column represents the mean ± SE of 7 rats. Saline: physiological saline challenge, OVA: OVA challenged control. †††p < 0.001: significantly different from the OVA challenged group (Student's t-test). ‡‡‡p < 0.001: significantly different from the OVA challenged group (Aspin-Welch test). **p < 0.01, ***p < 0.001: significantly different from the OVA challenged group (Dunnett test).

Fig. 5
Effects of olopatadine and prednisolone on the production of nerve growth factor (NGF) and vascular endothelial growth factor (VEGF) in the nasal lavage fluids (NALF) induced by the ovalbumin (OVA) challenge. NGF (A) and VEGF (B) levels in the NALF were measured 6 h after OVA challenge. Each column represents the mean ± SE of 6-7 rats. *p < 0.05: significantly different from the OVA challenged group (Dunnett test). †p < 0.05: significantly different from the OVA challenged group (Student's t-test). ‡p < 0.05: significantly different from the OVA challenged group (Aspin-Welch test).

References
1. Takahashi H, Ishida-Yamamoto A, Iizuka H. Effects of bepotastine, cetirizine, fexofenadine, and olopatadine on histamine-induced wheal-and flare-response, sedation, and psychomotor performance. Clin Exp Dermatol. 2004. 29:526–532.

2. Takahashi H, Zhang Y, Morita E. Evaluation of the antihistamine effects of olopatadine, cetirizine and fexofenadine during a 24 h period: a double-blind, randomized, crossover, placebo-controlled comparison in skin responses induced by histamine iontophoresis. Arch Dermatol Res. 2008. 300:291–295.
3. LaForce CF, Carr W, Tilles SA, Chipps BE, Storms W, Meltzer EO, Edwards M. Evaluation of olopatadine hydrochloride nasal spray, 0.6%, used in combination with an intranasal corticosteroid in seasonal allergic rhinitis. Allergy Asthma Proc. 2010. 31:132–140.

4. Segall N, Gawchik S, Georges G, Haeusler JM. Efficacy and safety of levocetirizine in improving symptoms and health-related quality of life in US adults with seasonal allergic rhinitis: a randomized, placebo-controlled study. Ann Allergy Asthma Immunol. 2010. 104:259–267.

5. Seppey M, Hessler C, Bruchez M, Savary M, Pécoud A. Facial thermography during nasal provocation tests with histamine and allergen. Allergy. 1993. 48:314–318.

6. Larbig M, Burtin B, Martin L, Stamm H, Luettig B, Hohlfeld JM, Krug N. Facial thermography is a sensitive tool to determine antihistaminic activity: comparison of levocetirizine and fexofenadine. Br J Clin Pharmacol. 2006. 62:158–164.

7. Sanico AM, Stanisz AM, Gleeson TD, Bora S, Proud D, Bienenstock J, Koliatsos VE, Togias A. Nerve growth factor expression and release in allergic inflammatory disease of the upper airways. Am J Respir Crit Care Med. 2000. 161:1631–1635.

8. Mosimann BL, White MV, Hohman RJ, Goldrich MS, Kaulbach HC, Kaliner MA. Substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide increase in nasal secretions after allergen challenge in atopic patients. J Allergy Clin Immunol. 1993. 92:95–104.

9. Shusterman D. Environmental nonallergic rhinitis. Clin Allergy Immunol. 2007. 19:249–266.
10. Matsune S, Ohori J, Yoshifuku K, Kurono Y. Effect of vascular endothelial growth factor on nasal vascular permeability. Laryngoscope. 2010. 120:844–848.

11. Yamashita T, Terada N, Hamano N, Kishi H, Kobayashi N, Kotani Y, Miura M, Konno A. Involvement of vascular endothelial growth factor in nasal obstruction in patients with nasal allergy. Allergol Int. 2000. 49:183–188.

12. Ohmori K, Hasegawa K, Tamura T, Miyake K, Matsubara M, Masaki S, Karasawa A, Urayama N, Horikoshi K, Kajita J, Hasegawa M, Taniguchi K, Komada T, Kawamoto Y. Properties of olopatadine hydrochloride, a new antiallergic/antihistaminic drug. Arzneimittelforschung. 2004. 54:809–829.
13. Yanni JM, Weimer LK, Sharif NA, Xu SX, Gamache DA, Spellman JM. Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch Ophthalmol. 1999. 117:643–647.

14. Roland PS, Ryan MW, Wall GM. Olopatadine nasal spray for the treatment of seasonal allergic rhinitis in patients aged 6 years and older. Expert Opin Pharmacother. 2010. 11:1559–1567.

15. Tamura T, Komai M. Effect of olopatadine hydrochloride, an anti-histamine drug, on rhinitis induced by intranasal instillation of toluene-2,4-diisocyanate in rats. Int Immunopharmacol. 2008. 8:916–921.

16. Shimizu T, Hirano H, Majima Y, Sakakura Y. A mechanism of antigen-induced mucus production in nasal epithelium of sensitized rats. A comparison with lipopolysaccharide-induced mucus production. Am J Respir Crit Care Med. 2000. 161:1648–1654.
17. Enomoto T, Lu HQ, Yin M, Sakoda T, Dake Y, Enomoto K, Ide T, Cheng L. Evaluation of the efficacy and safety of olopatadine and fexofenadine compared with placebo in Japanese cedar pollinosis using an environmental exposure unit. J Investig Allergol Clin Immunol. 2009. 19:299–305.
18. Okubo K, Okuda M, Magara H, Kaneko K. Olopatadine hydrochloride in children: efficacy and safety for perennial allergic rhinitis. Curr Med Res Opin. 2010. 26:1657–1665.

19. Roland PS, Marple BF, Wall GM. Olopatadine nasal spray for the treatment of allergic rhinitis. Expert Rev Clin Immunol. 2010. 6:197–204.

20. Tsumuro T, Alejandra Hossen M, Kishi Y, Fujii Y, Kamei C. Nasal congestion model in Brown Norway rats and the effects of some H1-antagonists. Int Immunopharmacol. 2006. 6:759–763.
21. Kaise T, Ohmori K, Sakakura Y, Ukai K. The effect of KW-4679, an antiallergic drug, on experimental allergic rhinitis in guinea pigs: effects on nasal blockage. Jpn J Pharmacol. 1995. 69:435–438.

22. Hansen I, Klimek L, Mösges R, Hörmann K. Mediators of inflammation in the early and the late phase of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2004. 4:159–163.

23. Rosenwasser L. New insights into the pathophysiology of allergic rhinitis. Allergy Asthma Proc. 2007. 28:10–15.

24. Sakai H, Enzaka J, Sakai-Oshita M, Chiba Y, Misawa M. Augmented venous responsiveness to leukotriene D4 in nasal septal mucosae of repeatedly antigen-challenged rats. Eur J Pharmacol. 2010. 644:215–219.
25. Sanico AM, Atsuta S, Proud D, Togias A. Dose-dependent effects of capsaicin nasal challenge: in vivo evidence of human airway neurogenic inflammation. J Allergy Clin Immunol. 1997. 100:632–641.

26. Miyake K, Horikoshi K, Ikeda Y, Ishii A, Karasawa A. Effects of olopatadine hydrochloride on the increase of histamine and peptide-leukotrienes concentrations in nasal lavage fluid following the antigen-antibody reaction in actively sensitized guinea pigs. Jpn J Pharmacol. 2001. 85:453–456.

27. Kaise T, Akamatsu Y, Ikemura T, Ohmori K, Ishii A, Karasawa A. Involvement of neuropeptides in the allergic nasal obstruction in guinea pigs. Jpn J Pharmacol. 2001. 86:196–202.

28. Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fcε receptor I expression. J Exp Med. 1998. 188:1135–1145.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download