Abstract
Pulseless electrical activity (PEA) is a clinical condition characterized by unresponsiveness and lack of palpable pulse in the presence of organized cardiac electrical activity and is caused by a profound cardiovascular insult (e.g., severe prolonged hypoxia or acidosis, extreme hypovolemia, or flow-restricting pulmonary embolus). Amyotrophic lateral sclerosis (ALS) is a disease that is characterized by progressive degeneration of all levels of the motor nervous system. Damage to the respiratory system and weakness of the muscles may increase the likelihood of an emergency situation occurring in patients with ALS while under general anesthesia. We report a case of PEA during the induction of general anesthesia in a patient with ALS who presented for dental treatment and discuss the causes of PEA and necessary considerations for general anesthesia in patients with ALS.
Pulseless electrical activity (PEA), also known as electromechanical dissociation, is a clinical condition characterized by unresponsiveness and lack of a palpable pulse in the presence of organized cardiac electrical activity [1]. PEA is found initially in about 55% of people in cardiac arrest and occurs when a major cardiovascular, respiratory, or metabolic derangement results in the inability of cardiac muscle to generate sufficient force in response to electrical depolarization. PEA is always caused by a profound cardiovascular insult (e.g., severe prolonged hypoxia or acidosis, extreme hypovolemia, or flow-restricting pulmonary embolus). The typical treatment approach is to initiate cardiopulmonary resuscitation (CPR) immediately and to identify and treat the underlying cause [123].
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a disease that is characterized by progressive degeneration of all levels of the motor nervous system [4]. The progressive degeneration of motor neurons causes symptoms of muscular weakness, lack of coordination, atrophy, fasciculations, spasticity, and hyperreflexia. This results in progressive asymmetric limb weakness and bulbar symptoms such as dysarthria (difficulty in speaking), dysphagia (difficulty in swallowing), and eventually respiratory muscle weakness (difficulty in breathing) [567]. As ALS progresses, atrophy and weakness may affect most skeletal muscles, including the diaphragm, costal muscles, and those of the pharynx and larynx. This may lead to a decrease in general condition and a higher risk of aspiration pneumonia due to dysphagia, an exaggerated change in the response to muscle relaxants, and delayed recovery of spontaneous breathing after general anesthesia, among other adverse side effects. Therefore, when considering anesthetic management in patients with ALS, careful monitoring and appropriate drug selection is necessary because the likelihood of emergency situations occurring is higher than that in healthy patients [78].
We report a case of PEA developing during the induction of general anesthesia in a patient with ALS who presented for dental treatment, and discuss the causes of PEA and necessary considerations for general anesthesia in patients with ALS.
A 58-year-old man presented to the Department of Advanced General Dentistry, Dankook University College of Dentistry in 2017 for comprehensive dental treatment. The patient was diagnosed with amyotrophic lateral sclerosis (ALS) after a spinal cord injury nine years prior to presentation in a traffic accident and no medication was prescribed. He was 178 cm tall and weighed 44 kg. He was unable to walk because of weakness of the lower limbs, and therefore presented in a wheelchair. He could not hold his head up because of atrophy of his neck muscles. There was a clear general decline in bodily function and he demonstrated slurring of speech, although communication was possible. He could not blink his eyes on instruction or freely swallow saliva because of dysphagia (Fig. 1). During the year prior to presentation, his dysphagia had become increasingly severe, resulting in aspiration pneumonia and admission. After that, he was taking a sticky liquid meal. There was no sensory deficit on examination.
Oral examination revealed the presence of proximal caries in the maxillary right first, second, and third molar and second premolar, and generalized periodontitis (Fig. 2A, B). A treatment plan including extraction, root canal treatment, and periodontal treatment was established. We decided to proceed with dental treatment under general anesthesia in the outpatient unit because the risk of reflux and lung aspiration due to the patient's swallowing dysfunction was expected to be high.
The patient had a history of severe hypotension after propofol infusion during general anesthesia induction performed at this center 4 years ago. A 4-hour dental treatment was performed while under general anesthesia with stable vital signs after atropine and ephedrine administration. Pre-anesthesia blood test and chest X-ray results were normal.
On the day of treatment, the patient presented after 8 hours of fasting and entered the operating room without receiving premedication. The patient was placed in a supine position on the dental chair and electrocardiography (EKG), non-invasive blood pressure monitoring (NIBP), and pulse oximeter monitoring were performed. An electroencephalogram (EEG)-entropy device (Entropy EasyFit Sensor disposable, Datex Ohmeda E-Entropy Module, GE Healthcare, Finland) was attached to the forehead of the patient to determine the anesthetic induction procedure and intraoperative anesthetic depth. The patient's initial vital signs were as follows: BP = 88/58 mmHg, heart rate = 82 beats/min, SpO2 = 98%, and electrocardiogram was normal.
A face mask was used to administer 4 L/min oxygen and nitrous oxide gas. After the patient's loss of consciousness, an 18-gauge intravenous catheter was inserted into the right arm and Hartman solution was administered. Next, 4% sevoflurane gas was added to maintain O2-N2O-sevoflurane (4 L/min-4 L/min-4% volume, respectively) via a face mask. A muscle relaxant (atracurium, 25 mg) was also administered via the intravenous line. The patient's BP dropped to 32/21 mmHg and the waveform as seen using the pulse oximeter was lost. In the EKG, sinus bradycardia (less than 40 beats/min) was persistent and the carotid pulse was lost. The end tidal CO2(ETCO2) dropped to 10 mmHg and our team diagnosed PEA (Fig. 3A, B). Since PEA is a cardiac arrest, our team performed cardiopulmonary resuscitation (CPR) according to the Advanced Cardiopulmonary Life Support (ACLS) guidelines [3910]. The anesthetic gas was immediately turned off and the patient was placed in the Trendelenburg position with 100% oxygen. Tracheal intubation was then performed with a 6.5-mm internal diameter endotracheal tube (Sewon Medical Corporation, Cheonan-si, Korea). After placement of the endotracheal tube, chest compression was applied at a rate of at least 100 compressions per minute continuously without pause for ventilation. The ventilation was given at 1 breath every 6 seconds (10 breaths per minute). Fluid was full dropped via the intravenous line and 0.5 mg epinephrine (0.01 mg/kg) was administered.
After 2 minutes of chest compressions, our team rechecked the vital signs of the patient. The blood pressure was 82/56 mmHg. The SpO2 and EKG graph showed a normal pattern. We judged that return of spontaneous circulation (ROSC) had been achieved, stopped CPR, and carefully monitored the patient. After confirming that the vital signs of the patient were stable, dental treatment was started. Anesthesia was maintained with 2 L/min O2, N2O, and 2% sevoflurane. During the treatment, blood pressure was maintained as low as 60–80/40–55 mmHg. The other vital signs were maintained as follows: heart rate, 75–95 beats/min; SpO2, 98%; and EEG-Entropy, 30–40. Ventilation was maintained for 2 hours without significant changes in BP or ETCO2. After completion of the treatment, intubation was removed after confirming that the patient's consciousness and spontaneous breathing were fully returned. Using a facial mask, 100% oxygen was administered and the patient was transferred to the recovery room. The oxygen saturation of the patient was stable and measured more than 95% when breathing atmospheric air, and there was no pattern of respiratory distress. The patient recovered after about an hour in the recovery room and returned home with his wife.
PEA is defined as the presence of spontaneous organized cardiac electric activity in the absence of blood flow sufficient to maintain consciousness and an absence of a rapid spontaneous return of adequate organ perfusion. It is characterized by the absence of a palpable pulse in an unconscious patient with organized electric activity other than ventricular tachyarrhythmia on EKG. The causes of PEA include (1) impairment of cardiac filling, (2) impaired pumping effectiveness of the heart, (3) circulatory obstruction, and (4) pathological vasodilation causing loss of vascular resistance and excess capacitance. These possible causes are remembered as the 5 Hs and the 5 Ts: hypovolemia, hypoxia, hydrogen ions (i.e., acidosis), hyper- or hypokalemia, and hypothermia and tablets or toxins, drug overdose, cardiac tamponade, tension pneumothorax, and thrombosis (pulmonary embolism), respectively [1311].
This case report is about PEA occurring in a patient with ALS while under general anesthesia. The first suspected cause of PEA was hypovolemia. As ALS progresses, difficulty in chewing and swallowing makes eating very difficult and increases the risk of choking or aspirating food. In later stages of the disorder, aspiration pneumonia can develop and maintaining a healthy weight can become a significant problem that may require the insertion of a feeding tube. Our patient is not yet equipped with a feeding tube but has a history of aspiration pneumonia. Consequently, he had restricted food intake and had lost a significant amount of weight (height, 178 cm; weight, 44 kg). Because of the 8-hour fasting period prior to general anesthesia, the body fluid volume of a patient is expected to be reduced. Therefore, when PEA occurred during induction of general anesthesia, our team performed volume replacement for management of hypovolemia at the same time as chest compressions. ROSC of patient recovered within 2 minutes because of the cause correction.
Second, sympathetic disturbances may have increased the PEA risk in our patient. ALS is a progressive neurodegenerative disorder that almost exclusively involves motor neurons. Although autonomic nervous systems are thought to be spared in ALS, their involvement, especially that of sympathetic neurons, has been suggested by subclinical and pathological findings, such as a smaller reduction in nocturnal blood pressure (BP), loss of correlation between BP and heart rate (HR), and degeneration of sympathetic neurons in the intermediolateral nucleus (IML) of the upper thoracic spinal cord [1213]. Asai et al. [13] reported that patients with ALS had reduced sympathetic activity in the terminal stages of disease, presumably due to neuronal loss in the IML, which may increase risk of sudden cardiac arrest. Oey et al. [14] investigated several aspects of autonomic function, such as muscle sympathetic nerve activity, vagal (baroreflex) control of heart rate, and cardiac function in patients with ALS and healthy volunteers. Patients with ALS had decreased spontaneous baroreflex sensitivity and reduced muscle sympathetic nerve activity [14]. Our patient also showed features of late ALS, such as asymmetric limb weakness and bulbar symptoms (e.g., dysarthria, dysphagia, and respiratory muscle weakness), and the initial blood pressure and heart rate were both very low. Therefore, sympathetic disturbances may have been related to the occurrence of PEA.
Because the sympathetic disturbance seen in patients with ALS is similar to that of neuraxial anesthesia, we performed ALS according to the cardiac arrest treatment guideline related to neuraxial anesthesia when PEA occurred. We discontinued sedation infusion and performed immediate tracheal intubation, followed by ventilation with 100% oxygen. Epinephrine 0.5 mg (0.01 mg/kg) was administered to treat the bradycardia. To resolve the hypovolemia, we changed the patient's position to the Trendelenburg position and performed a full fluid drop (Table 1) [10].
Care should be taken when inducing general anesthesia in ALS patients. Patients with ALS should be given sufficient hydration (approximately 500 ml intravenous fluid infusion) before anesthesia because dehydration due to fasting and likely dysphagia is severe. In addition, anesthetic concentrations should be gradually raised, while carefully monitoring vital signs, when providing anesthesia to patients with ALS.
Figures and Tables
Fig. 1
General appearance of the patient with amyotrophic lateral sclerosis. He is unable to walk and requires a wheelchair.

Fig. 2
Preoperative radiographic view of patient with amyotrophic lateral sclerosis. (A) Panoramic view, (B) periapical view.
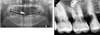
Fig. 3
Anesthetic record of patient with amyotrophic lateral sclerosis. The arrow indicates the vital signs when pulseless electrical activity occurs. (A) Oxygen saturation, heart rate, end tidal CO2 volume, and respiration rate, (B) blood pressure.

Table 1
Treatment of cardiac arrest associated with neuraxial anesthesia [10]

References
1. Myerburg RJ, Halperin H, Egan DA, Boineau R, Chugh SS, Gillis AM, et al. Pulseless electric activity: definition, causes, mechanisms, management, and research priorities for the next decade: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2013; 128:2532–2541.
2. Attin M, Tucker RG, Carey MG. In-Hospital Cardiac Arrest: An Update on Pulseless Electrical Activity and Asystole. Crit Care Nurs Clin North Am. 2016; 28:387–397.
3. Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015; 132:18 Suppl 2. S444–S464.
4. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011; 377:942–955.

5. Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015; 6:171.

6. Trivedi S, Tibrewala N, Balsara KP. Anaesthetic management of a patient with amyotrophic lateral sclerosis undergoing laparoscopic diaphragmatic pacing. Indian J Anaesth. 2015; 59:683–685.

7. Prabhakar A, Owen CP, Kaye AD. Anesthetic management of the patient with amyotrophic lateral sclerosis. J Anesth. 2013; 27:909–918.

8. Kim HS, Lee SY, Choi EH, Kim SO. Anesthetic Management of an Amyotrophic Lateral Sclerosis Patient Undergoing Dental Care in Daysurgery Center. J Korean Dent Soc Anesthesiol. 2013; 13:195–201.

9. Gabrielli A, O'Connor MF, Maccioli GA. Anesthesia advanced circulatory life support. Committee on Critical Care Medicine;2008.
10. Moitra VK, Gabrielli A, Maccioli GA, O'Connor MF. Anesthesia advanced circulatory life support. Can J Anaesth. 2012; 59:586–603.

11. Mehta C, Brady W. Pulseless electrical activity in cardiac arrest: electrocardiographic presentations and management considerations based on the electrocardiogram. Am J Emerg Med. 2012; 30:236–239.

12. Murata Y, Harada T, Ishizaki F, Izumi Y, Nakamura S. An abnormal relationship between blood pressure and pulse rate in amyotrophic lateral sclerosis. Acta Neurol Scand. 1997; 96:118–122.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download