Abstract
Glioblastoma, the most common primary malignant brain tumor in adults, is highly aggressive and associated with a poor prognosis. Bevacizumab, a monoclonal antibody against the vascular endothelial growth factor receptor, has increasingly been used in the treatment of recurrent glioblastoma. It has achieved excellent rates of radiographic response, but most patients will progress after only a few months. Upon recurrence, tumors may not enhance, secondary to vascular normalization. We describe four patterns of radiographic progression commonly associated with Bevacizumab failure: 1) Distant enhancing tumor, 2) Local tumor progression without enhancement, 3) Diffuse gliomatosis-like infiltration, and 4) Local or multifocal progression, with enhancement. Some have noted an increased incidence of distant or diffuse disease upon recurrence, suggestive of a transition to a more aggressive phenotype, but a review of the literature suggests there is no conclusive evidence that Bevacizumab treatment is associated with an increased rate of distant or diffuse recurrence.
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults, with an average annual incidence of approximately 3 per 100,000 individuals in the United States (US) [1]. Gliomas are defined as any tumor arising from glial or precursor cell origin, and include astrocytoma, oligodendroglioma, glioblastoma, ependymoma, mixed glioma, and other rarer histologies. Glioblastoma is the most aggressive form of glioma, categorized as World Health Organization (WHO) grade IV, and is associated with very poor prognosis, with median survival 14.6 to 16 months [23], with estimated 5 year survival rate of only 3.4% [4].
Standard first line management for newly diagnosed glioblastoma involves a multi-modality approach, including surgical resection, followed by radiation with concurrent and adjuvant temozolomide [2]. Even with optimal surgical resection and chemoradiation, however, essentially all patients' tumors will recur, and after recurrence, the 2 year survival is only 26% [2]. There is no standard management for recurrent disease.
Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), received accelerated approval from the US Food and Drug Adminsitration in 2009 based on promising trial results demonstrating improved response rates and 6-month progression-free survival (PFS) compared to historical controls [56]. Since then, Bevacizumab has increasingly been used in the treatment of recurrent glioblastoma, with high initial radiographic response and disease control rates [7]. The effects of Bevacizumab are transient, however, and most patients' tumors progress after a median time of 3–5 months [568].
Recent studies have categorized several patterns of radiographic disease progression in patients on Bevacizumab. Some hypothesize that these patterns of progression are characteristically different compared to patterns seen after other chemotherapeutic agents, and that they are suggestive of an adaptive phenotypic shift to a more aggressive histology. In this review, we will discuss the mechanism of action for Bevacizumab, key imaging features of Bevacizumab treatment response and treatment failure, and the data regarding whether these imaging features correspond to outcome or histology.
Bevacizumab is a recombinant humanized monoclonal antibody against the VEGF ligand. High grade gliomas express large quantities of VEGF, which, through a paracrine mechanism, promotes endothelial cell proliferation, survival and migration [9]. This leads to a propagation of disorganized vasculature with leaky blood brain barrier. Prior studies have shown that the degree of VEGF overexpression and subsequent angiogenesis is directly proportional to blood vessel density, tumor grade and overall outcome [910].
Inhibition of VEGF prunes abnormal blood vessels and “normalizes” vasculature into thin-walled vessels composed of a single layer of endothelial cells [11]. This vascular normalization is proposed to improve tissue oxygenation and drug delivery. The re-establishment of an intact blood-brain barrier also decreases vasogenic edema surrounding the tumor, often improving symptoms related to edema and mass effect, as well as allowing reductions in corticosteroid use.
In addition to normalizing vasculature, Bevacizumab is also thought to have direct anti-tumor activity against gliomas that express VEGF, and has been shown in animal models to alter glioblastoma cell migration [12]. Bevacizumab may also increase cell sensitization to other cytotoxic agents, making it an effective therapeutic agent to use in combination with concurrent chemotherapy or radiation [1314].
Bevacizumab is approved for use in a variety of malignancies characterized by rapid angiogenesis: colorectal cancer, non-small-cell lung cancer, breast cancer, and glioblastoma. It received accelerated approval from the US Food and Drug Administration in 2009 for use in glioblastoma, based on phase II trial results demonstrating improved response rates and 6-month PFS compared to historical controls [56]. Bevacizumab has been used both alone and in combination with radiation and chemotherapy for recurrent glioblastoma.
Multiple studies using Bevacizumab in combination with chemotherapy [1516171819202122], as well as two phase II trials using Bevacizumab alone [56], have demonstrated both radiographic responses and increased PFS in patients with recurrent glioma, relative to historical data in patients who received chemotherapy alone. These responses are transient, however, and other recent prospective comparisons of Bevacizumab with other chemotherapeutic regimens have shown no significant increase in overall survival (OS) [232425].
The mechanisms of Bevacizumab failure are likely complex and multifactorial. Over time, tumors demonstrate adaptive upregulation of alternative angiogenic pathways [26]. Animal models of inhibited angiogenesis through VEGF blockade demonstrate that over time, glioma cells co-opt and invade along normal cerebral vasculature, leading to distant satellite tumor formation [1027]. Radiographically, this concept is demonstrated through development of distant recurrence, remote from the site of the index lesion. Additionally, with normalized vasculature and more intact blood brain barriers, recurrence often does not enhance. It has been previously suggested in the literature that this non-enhancing, distant recurrence indicates a more aggressive or invasive disease phenotype [711], but whether this is actually the case is still under debate.
Historically, the evaluation of treatment response had been made using the Macdonald criteria [28], a method that uses post-contrast images and clinical features to evaluate the degree of disease response or progression. Over time, however, as the imaging of glioblastoma has become more nuanced from a clinical perspective, the Macdonald criteria has largely been replaced by the Response Assessment in Neuro-Oncology (RANO) criteria (Table 1) [29]. The RANO criteria builds upon the previously established Macdonald criteria and takes into account both enhancing and non-enhancing disease progression, and also accounts for corticosteroid use, as steroid use may confound the extent of enhancement and fluid-attenuated inversion recovery (FLAIR) hyperintensity. Complete response requires that the patient be completely off, or on only a physiologic replacement dose, of corticosteroids.
Imaging features of treatment response to Bevacizumab include decreased contrast enhancement on T1 post-contrast sequences and stable to decreased vasogenic edema on FLAIR sequences (Fig. 1). Normalization of vasculature decreases contrast extravasation into extra-cellular spaces, leading to a decrease in post-contrast enhancement [30]. These imaging changes have been documented as early as 14 days [15].
Accurate assessment of treatment response is important because several studies have shown radiographic response to Bevacizumab correlates with PFS or OS. Although these studies demonstrate a correlation between imaging response and survival, they differ in their exact correlations. Shapiro et al. [31] demonstrated that those with complete or partial radiographic response after irradiation and Bevaciumab had longer PFS than those with stable imaging after treatment (9.6 months vs. 4.6 months). In that study, radiographic patterns of progression and location of recurrence had no correlation with survival. In contrast, the prospective randomized BELOB trial [23] and retrospective study by Prados et al. [32], which found that radiographic response to treatment was the only correlate to OS.
Glioblastoma is a highly infiltrative tumor and impossible to resect in its entirety. As a result, the majority of recurrences occur locally around the resection cavity. Bevacizumab is believed to alter the radiographic appearance of recurrence, and some believe may increase the incidence of diffuse and distant tumor recurrence. Several prior studies have categorized various patterns of recurrence on Bevacizumab [78313334]. Although the categorization methods used in these studies all address location and enhancement of recurrent disease, the categorization systems themselves are all different.
Norden et al. [7] described three main patterns of recurrence based on the Macdonald criteria for disease progression: 1) Distant recurrence: new foci of enhancement distant from the original area of enhancing tumor; 2) Local recurrence: increased enhancement in contiguity with the original tumor mass; and 3) Diffuse recurrence: at least a 25% increase in area of abnormal FLAIR hyperintensity, but local enhancing tumor mass remained stable. Using this system, Norden studied 26 patients with GBM treated with Bevacizumab and 19 control patients with GBM who did not receive Bevacizumab, and found that the patterns of recurrence were local 62%, diffuse 15%, distant 15%, and no progression 2% in the Bevacizumab group and local 68%, diffuse 16%, distant 5%, and no progression 11% in the control group. The study found no significant difference in the pattern of recurrence between the Bevacizumab-treated patients relative to a control population. This study did not investigate whether patterns of Bevacizumab failure correlated with PFS or OS.
Shapiro et al. [31] studied patterns of progression in patients undergoing Bevacizumab with concurrent hypofractionated stereotactic radiation therapy, and specifically, whether recurrence enhanced, and whether the recurrence was within the radiation field. Their study found that 71.4% of patients had enhancing recurrent disease and 28.6% of patients had non-enhancing recurrent disease at the time of progression. The study additionally found that pattern of enhancement and location of recurrence (whether in-field or out-of-field) did not correlate with OS or PFS.
Zuniga et al. [34] retrospectively reviewed 55 patients with recurrent high grade glioma treated with Bevacizumab and irinotecan, and also specified three main patterns of recurrence based on a modified Macdonald criteria: 1) Distant recurrence: at least one new focus of enhancement observed outside of a 2 cm margin surrounding the resection cavity; 2) Local recurrence: increase in at least 25% in the maximal cross-sectional area of enhancement within a 2 cm margin around the resection cavity; and 3) Diffuse recurrence: recurrence on FLAIR not concordant with increased areas of enhancement. Their study found a high number of patients with a diffuse pattern of progression, where the progression on FLAIR was discordant with the progression of enhancing disease, and suggested that with Bevacizumab therapy, FLAIR sequences may more accurately reflect the burden of infiltrative tumor. Their study did not correlate patterns of progression with OS or PFS.
Pope et al. [8] expanded upon prior classification systems and described four patterns of disease progression: 1) Local progression: focus of enhancement or non-enhancing tumor at or within 3 cm of the primary resection cavity; 2) Distant progression: single new focus of enhancement or a qualitative assessment of recurrence centered more than 3 cm from the primary resection cavity; 3) Diffuse recurrence: recurrence either centered or extending more than 3 cm from the primary site or margin of resection cavity with 50% or greater of the margin of the recurrent tumor qualitatively assessed as poorly defined; and 4) Multifocal: more than one lesion site with each lesion having a mostly or complete well-defined border with intervening areas of normal brain signal. Pope compared disease patterns at baseline and at progression for patients enrolled in the BRAIN trial, who were randomized into two groups who were given Bevacizumab alone or Bevacizumab with irinotecan. Their study found that in the Bevacizumab alone group (n=67), of the 48 patients who had local disease at baseline, 37 continued to demonstrate local disease at progression, but 11 shifted to diffuse disease. The 1 patient with distant disease at baseline continued to demonstrate distant disease at progression, and similarly the 14 patients who demonstrated diffuse disease at baseline continued to demonstrate diffuse disease at progression. Of the 4 patients who demonstrated multifocal disease at baseline, 3 continued to demonstrate multifocal disease, but 1 shifted to a diffuse pattern. In patients treated with Bevacizumab and irinotecan (n=57), of the 40 people who demonstrated a local pattern of disease at baseline, 18 continued to demonstrate a local pattern but 22 switched to a diffuse pattern. Of the 12 people who demonstrated a diffuse pattern at baseline, 11 continued to demonstrate a diffuse pattern, but 1 shifted to a multifocal pattern. And of the 5 people who demonstrated a multifocal pattern at baseline, 1 remained multifocal at progression, 1 shifted to a distant pattern, and 3 shifted to a diffuse pattern.
In summary, the study showed that most patients did not experience a shift in tumor pattern at time of progression (82% in the Bevacizumab group and 53% in the Bevacizumab with irinotecan group). Additionally, the pattern of progression had no impact on objective response, PFS and OS.
Nowosielski et al. [33] most recently employed four patterns of tumor progression in her study of whether patterns of progression correlated with outcome in patients with GBM: 1) T2-diffuse: tumor progression by either a complete decrease in contrast-enhancement or only a few faintly speckled enhancing lesions, with a homogeneous increase in T2 signal at time of progression; 2) cT1 flare-up: initial decrease in enhancement, followed by a “flare up” of enhancing disease at time of tumor progression, with stable T2 signal; 3) Primary nonresponder: stable, increased or new enhancing lesions distant from the original tumor site at first follow-up imaging after commencement of therapy, with stable or increased T2 signal; and 4) T2-circumscribed: complete decrease in enhancement or a few faintly speckled enhancing lesions, but bulky and inhomogeneous T2 progression with sharp borders. Their study found 15/83 (18%) had a T2-diffuse pattern, 35/83 (42%) had a cT1 flare-up pattern, 16/83 (19%) had a primary nonresponder pattern and 17/83 (21%) had a T2-cirumscribed pattern. The T2-diffuse pattern had a longer OS (17.7 months) after initiation of Bevacizumab than the other patterns. After progression on Bevacizumab, T2-diffuse and cT1 flare-up patients survived longer (4.8 and 4.6 months) than the primary non-responders or T2-circumscribed patients (3.0 and 1.6 months). Additionally, the study found that the time to development of a T2-diffuse or cT1 flare-up pattern of progression was longer than for the development of primary non-responder or T2-circumscribed progression. Thus the authors proposed that the T2-diffuse subtype may represent a different glioblastoma biology that takes longer to develop and also demonstrates a longer overall survival after Bevacizumab therapy.
Each of the above classification system has its own set of limitations. Specifically, Norden, Shapiro and Zuniga's categorizations, based off of the Macdonald criteria, do not take into account the possibility of distant, localized, non-enhancing recurrence. Pope and Nowosielski's systems contain specific criteria that leave some tumors outside of the classification scheme. Combining the categorization techniques of these studies with our own institutional experience, we summarize four main imaging patterns of treatment failure frequently associated with Bevacizumab therapy:
1) Distant enhancing tumor: although the site of index lesion demonstrates decreased FLAIR hyperintensity and decreased contrast enhancement, consistent with treatment response, there is the development of new, distant enhancing tumor, with or without subependymal spread of disease (Fig. 2, 3).
2) Local tumor progression at site of original disease, without enhancement: development of an enlarging area of reduced diffusion and/or FLAIR hyperintensity locally at the site of original disease, with no contrast enhancement (Fig. 4).
3) Diffuse gliomatosis-like tumor infiltration: development of a diffuse FLAIR hyperintensity in a gliomatosis-like pattern, with minimal contrast enhancement (Fig. 5).
4) Local or multifocal progression, with enhancement: development of increased FLAIR hyperintensity with increased contrast-enhancement either locally or at multiple sites (Fig. 6).
Recognizing these various patterns of Bevacizumab failure will provide more sensitive evaluation of progressive disease, and will help differentiate findings of progressive disease from complications such as hemorrhage or infarction.
Bevacizumab, a monoclonal antibody against the VEGF ligand, has increasingly been used in the treatment of recurrent glioblastoma. It has achieved excellent rates of radiographic response, often demonstrating a remarkable decrease in tumor enhancement and FLAIR hyperintensity, owing to its mechanism of vascular normalization. Several studies have shown that degree of radiographic response correlates with either OS or PFS. The effects of Bevacizumab are transient, however, and most patients progress after only a few months. Owing to its mechanism of action, recurrence on Bevacizumab is often non-enhancing, and some have noted an increased incidence of distant or diffuse disease, suggestive of a transition to a more aggressive phenotype. We describe four patterns of radiographic progression commonly associated with Bevacizumab treatment failure: 1) Distant enhancing tumor, 2) Local tumor progression without enhancement, 3) Diffuse gliomatosis-like infiltration, and 4) Local or multifocal progression, with enhancement. Although it has been suggested, there is no conclusive evidence that Bevacizumab treatment is associated with an increased rate of distant or diffuse recurrence. There is also no definite correlation between pattern of progression and survival. Recognizing these patterns of progression, however, will allow for improved diagnosis of treatment failure and allow for differentiation of progressive disease from other treatment complications.
Figures and Tables
Fig. 1
Pre-treatment and post-treatment axial FLAIR (A and B, respectively) demonstrate decreased vasogenic edema post-treatment (arrows). Pre- and post-treatment axial T1 post-contrast (C and D, respectively) demonstrate decreased contrast enhancement (arrowheads). These findings are consistent with treatment response. FLAIR, fluid-attenuated inversion recovery.
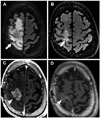
Fig. 2
Pre-treatment and post-treatment axial (A and B, respectively) FLAIR images demonstrate decreased vasogenic edema post-treatment (arrows). Pre- and post-treatment axial T1 post-contrast images through the site of the index lesion (C and D, respectively) demonstrate decreased enhancement of disease (arrowheads). Pre-and post-treatment axial T1-post contrast images through a location distant from the index lesion (E and F, respectively) demonstrate a new distant enhancing tumor post-treatment (low arrow). Pattern of treatment failure is consistent with distant enhancing tumor. FLAIR, fluid-attenuated inversion recovery.
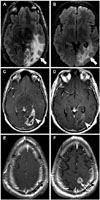
Fig. 3
Pre-treatment and post-treatment axial FLAIR images (A and B, respectively) demonstrate decreased vasogenic edema post-treatment (arrows). Pre- and post-treatment axial T1 post-contrast through the level of the index lesion (C and D, respectively) demonstrate decreased enhancement at the site of disease (arrowheads). Pre-and post-treatment axial T1-post contrast images through axial levels cranial to the index lesion (E and F, and G and H, respectively) demonstrate new distant enhancing nodules in a pattern consistent with subependymal spread of disease (long arrows). Pattern of treatment failure is consistent with distant enhancing tumor. FLAIR, fluid-attenuated inversion recovery.
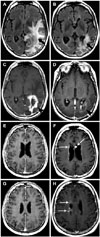
Fig. 4
Pre-treatment and post-treatment axial FLAIR (A and B, respectively) demonstrates increased mass-like FLAIR hyperintensity post-treatment (arrows). Pre- and post-treatment axial T1 post-contrast through the level of the index lesion (C and D, respectively) demonstrate decreased enhancement of the original disease (arrowheads). Pre- and post-treatment axial DWI (E and F, respectively), and pre- and post-treatment ADC maps (G and H, respectively) demonstrate increased mass-like area of reduced diffusion post-treatment (long arrows). Pattern of treatment failure is consistent with local tumor progression without enhancement. FLAIR, fluid-attenuated inversion recovery; DWI, diffusion weighted image; ADC, apparent diffusion coefficient.
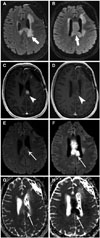
Fig. 5
Pre- and post-treatment axial T1 post-contrast images (A and B, respectively) demonstrate decreased tumor enhancement post-treatment (arrowheads). Multiple pre-treatment (C, E, G, and I) and post-treatment (D, F, H, and J) axial FLAIR images demonstrate development of a diffuse infiltrative FLAIR hyperintensity in a gliomatosis-like pattern (arrows). Pattern of treatment failure is consistent with diffuse gliomatosis-like tumor infiltration. FLAIR, fluid-attenuated inversion recovery.
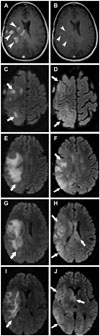
Fig. 6
Pre- and post-treatment axial FLAIR (A and B, respectively) demonstrates increased mass-like FLAIR hyperintensity post-treatment (arrows). Pre- and post-treatment axial DWI (C and D, respectively), and pre- and post-treatment ADC map (E and F, respectively) demonstrate an increased mass-like area of reduced diffusion (long arrows). Pre- and post-treatment axial T1 post-contrast images through the level of the index lesion (G and H, respectively) demonstrate increased enhancement. Pattern of treatment failure is consistent with local or multifocal progression with enhancement (arrowheads). FLAIR, fluid-attenuated inversion recovery; DWI, diffusion weighted image; ADC, apparent diffusion coefficient.
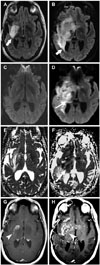
Table 1
RANO criteria for tumor response, which takes into account both MRI and clinical factors

Adapted from Wen et al. J Clin Oncol 2010;28:1963-72 [29]. Criteria for response assessment incorporating MRI and clinical factors [29]. All measurable and nonmeasurable lesions must be assessed using the same techniques as at baseline. Stable doses of corticosteroids include patients no on corticosteroids. RANO, response assessment in neuro-oncology; FLAIR, fluid-attenuated inversion recovery
References
1. Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006; 2:494–503.

2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.

3. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013; 31:4085–4091.

4. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014; 16:Suppl 4. iv1–iv63.

5. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009; 27:4733–4740.

6. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009; 27:740–745.

7. Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008; 70:779–787.

8. Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011; 76:432–437.

9. Kargiotis O, Rao JS, Kyritsis AP. Mechanisms of angiogenesis in gliomas. J Neurooncol. 2006; 78:281–293.

10. Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000; 2:306–314.

11. de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010; 12:233–242.

12. Lu KV, Chang JP, Parachoniak CA, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012; 22:21–35.

13. Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008; 8:579–591.

14. Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011; 76:87–93.

15. Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006; 66:1258–1260.

16. Ali SA, McHayleh WM, Ahmad A, et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: a series of 13 cases. J Neurosurg. 2008; 109:268–272.

17. Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008; 112:2267–2273.

18. Kang TY, Jin T, Elinzano H, Peereboom D. Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol. 2008; 89:113–118.

19. Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009; 110:173–180.

20. Thompson EM, Dosa E, Kraemer DF, Neuwelt EA. Treatment with bevacizumab plus carboplatin for recurrent malignant glioma. Neurosurgery. 2010; 67:87–93.

21. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007; 13:1253–1259.

22. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007; 25:4722–4729.

23. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014; 15:943–953.

24. Brandes AA, Finocchiaro G, Zagonel V, et al. AT-11. Final results from the randomized phase II trial avareg (ML25739) with bevacizumab (BEV) or fotemustine (FTM) in recurrent GBM. Neuro Oncol. 2014; 16:suppl 5. v10.
25. Wick W, Brandes AA, Gorlia T, et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol. 2016; 34:suppl. abstract 2001.

26. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008; 8:592–603.

27. Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001; 61:6624–6628.
28. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280.

29. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28:1963–1972.

30. Claes A, Gambarota G, Hamans B, et al. Magnetic resonance imaging-based detection of glial brain tumors in mice after antiangiogenic treatment. Int J Cancer. 2008; 122:1981–1986.

31. Shapiro LQ, Beal K, Goenka A, et al. Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int J Radiat Oncol Biol Phys. 2013; 85:636–642.

32. Prados M, Cloughesy T, Samant M, et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011; 13:143–151.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download