Abstract
Background
The purpose of this study was to investigate plasma levels of endostatin and vascular endothelial growth factor (VEGF) in normal subjects and in patients with pituitary adenoma and to evaluate change in these levels following stereotactic radiosurgery (SRS) for pituitary adenoma.
Methods
Peripheral venous blood was collected from five patients with pituitary adenoma before SRS using Gamma Knife and at the 1 week and 1 month follow-up visits. Plasma endostatin and VEGF levels were measured using commercially available enzyme-linked immunosorbent assay kits. Peripheral blood samples were obtained from 10 healthy volunteers as controls.
Results
Mean baseline plasma endostatin level (105.3 ng/mL, range, 97.0-120.2 ng/mL) in patients with pituitary adenoma was higher than that of the healthy controls (86.6 ng/mL, range, 71.3-98.2 ng/mL) (p=0.001). Mean plasma VEGF level was 89.5 pg/mL (range, 24.1-171.8 pg/mL) in patients with pituitary adenoma at baseline and 29.3 pg/mL (range, 9.2-64.3 pg/mL) in the control group (p=0.050). Plasma endostatin level changed to 106.6 ng/mL 1 week after SRS and decreased to 95.9 ng/mL after 1 month. Plasma VEGF level following SRS decreased to 74.1 pg/mL after 1 week and 79.0 pg/mL after 1 month. There was a trend toward decreased plasma endostatin and VEGF concentrations 1 month after SRS compared to baseline levels (p=0.195, p=0.812, respectively).
Pituitary adenoma accounts for 10-20% of all intracranial tumors, and is a highly angiogenic tumor that secretes vascular endothelial growth factor (VEGF) [12]. Angiogenesis or neovascularization plays an important role in tumor growth, which is determined by angiogenesis stimulatory factor and its inhibitors, VEGF and endostatin. VEGF is secreted by the normal pituitary glands and all types of adenomas may be involved in pituitary tissue growth [345]. Endostatin is one of a number of endogenously generated anti-angiogenic protein fragments that have anti-tumoral activity in a murine model [6].
Surgical resection is the initial treatment for pituitary adenoma; however, delayed recurrence after complete resection can occur in 24-80% of cases [7]. Stereotactic radiosurgery (SRS) has been used for pituitary adenoma in cases when the tumor has recurred or was not completely removed. Although pituitary adenoma control rates after SRS are high, analysis of angiogenic factors after Gamma Knife radiosurgery (GKS) may be needed to understand the anti-tumoral and anti-angiogenic effect of GKS [2].
Angiogenesis plays an important role in the progression of pituitary adenomas; however, no study has evaluated plasma endostatin and VEGF levels in patients with pituitary adenoma before and after radiosurgery. In this study, we evaluated plasma endostatin and VEGF levels in patients with pituitary adenoma before and after SRS.
Five patients with pituitary adenoma, all of whom provided written informed consent, were enrolled in this prospective protocol that was reviewed and approved by the Institutional Review Board of our hospital (No. KNUH 2012-07-021-001). All patients underwent SRS following surgical resection. SRS was considered for patients with recurrent or residual pituitary adenoma after an initial transsphenoidal resection. Three patients experienced three operations and two patients underwent one surgery before SRS. All pituitary adenomas were pathologically proven to be non-functioning pituitary adenomas. Peripheral blood samples donated from 10 volunteers wi-thout known malignancy or pregnancy were used as controls.
Peripheral venous whole blood was obtained before radiosurgery (baseline sample) and 1 week and 1 month after SRS. Blood samples from study participants were collected in ethylenediaminetetraacetic acid tubes and centrifuged at 3,200 rpm for 10 minutes at 4℃. The supernatant including the plasma fraction was transferred to a microtube and frozen im-mediately at -80℃ until analysis. The analysis was performed with commercially available enzyme-linked immunosorbent assays kits (Quantikine Human VEGF Immunoassay, R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. All patient samples collected were assayed simultaneously.
SRS was carried out using a Gamma Knife Model C (Elekta AB, Stockholm, Sweden). T1-, T2-, and enhanced T1-weighted magnetic resonance imaging (MRI) with a slice thickness of 2 mm was used for three-dimensional reconstructions and treatment planning. The MRIs were transferred to a workstation for post-processing and analysis. The Gamma Plan system was used to determine the GKS for all patients. Multiple small isocenters were used to deliver a highly conformal dose to the tumor. Tumor volume ranged from 1.1 to 4.9 mL (mean, 2.3 mL). Mean marginal dose was 13.6 Gy (range, 12-15 Gy). The 50% isodose line was used as the margin in all patients. Maximum radiation dose was 24-30 Gy (mean, 27.2 Gy). The size of each tumor before and 6 months and 1 and 2 years after GKS was measured retrospectively using the Gamma Plan workstation and a picture archiving communication system. Tumor progression was defined as an increase in tumor volume of at least 10%. Tumor regression was defined as at least a 10% decrease in tumor volume. Tumors that were ±10% of their original volume were defined as stable [7].
All analyses were performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Because the data were not distributed normally, they were analyzed using the Wilcoxon's rank-sum test, the Mann-Whitney U-test. Correlations were assessed using the nonparametric Spearman's rank correlation analysis. p-values<0.05 were considered significant.
Of the five consecutive patients enrolled, three were men and two were women (mean age, 46.6 years; range, 40-55 years). Six men and four women with a mean age of 47.6 years (range 30-55 years) were included as controls. Blood samples were drawn from five pituitary adenoma patients before SRS and 1 week after SRS, and 1 month after SRS. The patient and tumor characteristics as well as the radiosurgical parameters, are summarized in Table 1. Four tumors had decreased in size and one tumor had increased in size at the 2 year MR follow-up (Table 2). No radiosurgical complications were noted.
Mean plasma endostatin and VEGF concentrations in controls were 86.6 ng/mL and 29.3 pg/mL, respectively. There was no significant differences in mean plasma endostatin as well as VEGF levels between men and women. There was no correlation between mean plasma concentrations of endostatin and VEGF levels and age.
Mean baseline plasma endostatin level was 105.3 ng/mL (range, 97.0-120.2 ng/mL) in patients with pituitary adenoma, which was significantly higher than that in the control group (mean, 86.6 ng/mL; range, 71.3-98.2 ng/mL) (p=0.001) (Fig. 1). There was no significant difference in plasma endostatin levels between men and women (p=0.638). No correlation was found between endostatin level and age (p=0.873) or tumor size (p=0.391). Mean plasma VEGF level was 89.5 pg/mL (range, 24.1-171.8 pg/mL) at baseline in patients with pituitary adenoma and 29.3 pg/mL (range, 9.2-64.3 pg/mL) in the control group (p=0.050) (Fig. 2). Plasma VEGF levels in patients with pituitary adenoma were higher than those in the control group; however, no significant difference was observed between the two groups. No correlation was found in plasma VEGF levels between men and women (p=0.863). No correlation was observed between VEGF level and age (p=0.285) or tumor size (p=0.391).
The effect of SRS for pituitary adenomas was evaluated by sequential measurement of plasma endostatin and VEGF concentrations before SRS and 1 week and 1 month after SRS. Mean plasma endostatin level changed to 106.6 ng/mL 1 week after SRS and then dropped to 95.9 ng/mL after 1 month (Fig. 5). There was a trend toward decreased plasma endostatin concentrations 1 month after SRS compared to baseline levels (p=0.195). Plasma endostain concentration 1 month after SRS was still elevated over control levels, even though it was not significantly different from control levels (p=0.140).
Mean plasma VEGF level following SRS decreased to 74.1 pg/mL at 1 week and 79.0 pg/mL at 1 month (Fig. 6). Mean plasma VEGF level 1 month after SRS decreased compared to pretreatment level; however, there was no significant difference from baseline levels (p=0.812). Plasma VEGF level 1 month after SRS was still significantly increased over controls (p=0.033).
Four patients with a decrease in tumor size experienced a constant decrease in endostatin and VEGF concentrations. One patient with an enlarged pituitary adenoma after SRS had an elevated VEGF level and a slightly decreased endostatin level (Fig. 7). This patient underwent surgical resection for a recurrent tumor 2 years after SRS. No patient had radiation necrosis following SRS.
Angiogenesis is essential for the development and progression of brain tumors. Angiogenesis is thought to be induced by an imbalance between pro- and anti-angiogenic factors. VEGF is the most potent angiogenic factor known so far and endostatin is a potent endogenous angiogenesis inhibitor [389]. Endostatin and VEGF have recently been implicated in the pathophysiology of pituitary adenoma. VEGF is a tyrosine kinase that plays an important role in angiogenesis and modulation of vascular permeability. VEGF-A binds with high specificity to VEGF receptor-1 and VEGF receptor-2 on vascular endothelial cells. These receptors modulate downstream signaling pathways affecting various cellular processes [10]. Increased plasma levels of VEGF are observed in patients with pituitary adenomas, compared to those in controls were detected [4]. Endostatin is a cleaved fragment of collagen XVIII, and inhibits migration of endothelial cells and induces endothelial cell apoptosis [6]. Plasma endostatin concentrations are significantly higher in patients with pituitary adenoma compared to those in controls [3].
Patients with pituitary adenoma usually undergo surgical resection; however, complete resection is often difficult. SRS is an effective and well-tolerated management option for recurrent or residual pituitary adenoma [2]. Recurrent or residual tumors are related to significant morbidity over the lifetime of a patient. Monitoring tumor response with biomarkers may be useful to improve long-term treatment outcomes of patients who undergo SRS for pituitary adenoma.
We evaluated plasma endostatin and VEGF levels in patients with pituitary adenoma before/after SRS and in a control group. We sought to understand angiogenic mechanisms regulating pituitary adenomas and the anti-angiogenic effect of SRS by studying changes in plasma endostain and VEGF levels in patients with pituitary adenoma undergoing SRS. A better understanding of the role of abnormal angiogenesis in patients with pituitary adenoma may lead to improved patient management and novel therapeutic options.
Plasma endostain and VEGF levels were significantly higher in patients with pituitary adenoma at baseline in comparison to the controls in this study, which is consistent with those of previous studies [34]. Although several studies have found that abnormal local expression of endostain and VEGF plays a role in angiogenesis in pituitary adenomas, the cause of increased endostain and VEGF still remains unclear [13456]. Feldman et al. [1112] found that circulating endostatin and VEGF levels increase in patients with clear cell renal cancer and colorectal cancer with liver metastasis. They suggested that the correlation between circulating endostatin and VEGF may be associated with secretion of proteases that cleave endostatin from collagen XVII, both from tumor and endothelial cells. VEGF upregulates the release of proteases. Elevated plasma endostain levels may be a defense mechanism to protect the host from angiogenesis by VEGF [13]. Angiogenesis inhibitors are effectively suppress growth of experimental pituitary adenoma [14].
Plasma VEGF is detectable in the peripheral blood of normal healthy volunteers [9]. The origin and biologic role of VEGF in healthy subjects are unknown; however, this finding suggests a role for endothelial mitogens in the maintenance of physiologic endothelial integrity [8]. In healthy controls, a significant negative correlation between circulating endostatin and VEGF levels was found [3]. It results from balance mechanism between angiogenic and anti-angiogenic factors. Our results showed that VEGF and endostatin levels in the peripheral blood in patients with pituitary adenoma increased at the same time. Gruszka et al. [3] suggested that simultaneous elevation of endostatin and VEGF may attenuate the pro-angiogenic action of VEGF and could be responsible for the ra-ther weak neovascularization of pituitary adenomas. Pituitary adenomas are mostly benign and have lower vascular density than that of non-tumorous glands. Low microvascular density or inhibited angiogenesis may be a partial explanation for the relatively low growth potential observed in pituitary tumors [3515].
The plasma endostatin and VEGF levels in the patients with pituitary adenoma dropped 1 month after SRS but were still elevated over control levels, confirming abnormal angiogenesis in pituitary pathophysiology. This finding suggests that the natural history of a pituitary adenoma is related to disruption of endostatin and VEGF expression. Our result suggests that Gamma Knife-irradiated pituitary adenoma tissue has significantly less angiogenic activity than that of a previously untreated pituitary adenoma. Our results support the anti-angiogenic effects of SRS, which may be important for treating pituitary adenomas.
Unlike four patients, one patient experienced increased endostain and VEGF levels after SRS. That patient experienced a tumor recurrence 2 years after SRS. Systemic expression of endostatin and VEGF may be related to the development, progression in pituitary adenoma patients. O'Sullivan et al. [16] reported that the 5- and 10-year actuarial rates of recurrence or growth of a residual adenoma after resection are 24.4% and 51.5%, respectively. Monitoring peripheral blood biomarkers in patients with pituitary adenoma may be valuable for those with highly angiogenic tumors, which tend to recur. Serial plasma endostatin and VEGF measurements may be a useful to monitor the treatment efficacy of SRS for angiogenic tumor cells in the future.
The main limitations of our study were the small number of patients and the short follow-up period. Sufficient plasma endostatin and VEGF data in a control group and abnormal levels in patients with pituitary adenoma are needed to use them as biomarkers and a diagnostic tool to monitor the tumor response after SRS. A controlled systematic prospective study with a longer follow-up is necessary to investigate the anti-angiogenic effect of SRS in patients with pituitary adenoma.
In conclusions, plasma endostatin and VEGF levels in patients with pituitary adenoma are elevated over controls at baseline and decrease 1 month after SRS. This was a preliminary study on the potential association between plasma endostatin and VEGF levels and pituitary adenoma. Our results provide evidence for further work related to determining whether plasma endostatin and VEGF levels could be useful to monitor the tumor response after SRS.
Figures and Tables
Fig. 1
Plasma concentrations of endostatin in controls and patients with pituitary adenoma (p=0.001).

Fig. 2
Plasma concentrations of vascular endothelial growth factor (VEGF) in controls and patients with pituitary adenoma (p=0.050).

Fig. 3
Correlation between serum concentrations of endostatin and vascular endothelial growth factor (VEGF) in the control group (r=-0.188, p=0.603).

Fig. 4
Correlation between serum concentrations of endostatin and vascular endothelial growth factor (VEGF) in patients with pituitary adenoma (r=0.500, p=0.391).

Fig. 6
Plasma concentrations of vascular endothelial growth factor (VEGF) before and after stereotactic radiosurgery (SRS).

Fig. 7
Enhanced T1-weighted (A) magnetic resonance image (MRI) obtained in a 46-year-old man with a pituitary adenoma. Stereotactic radiosurgery (SRS) was performed with a margin dose of 14 Gy to the 50% isodose line. The MRI obtained 2 years after SRS shows an increase of tumor volume from 2.5 mm3 to 4.8 mm3 (B). Plasma endostatin concentrations slightly decreased and vascular endothelial growth factor increased 1 month after SRS.
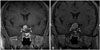
Table 1
The patient and tumor characteristics and radiosurgical parameters
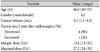
Table 2
Demographic and clinical characteristics of 5 pituitary adenoma patients

References
1. Sánchez-Ortiga R, Sánchez-Tejada L, Moreno-Perez O, Riesgo P, Niveiro M, Picó Alfonso AM. Over-expression of vascular endothelial growth factor in pituitary adenomas is associated with extrasellar growth and recurrence. Pituitary. 2013; 16:370–377.

2. Sheehan JP, Starke RM, Mathieu D, et al. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J Neurosurg. 2013; 119:446–456.

3. Gruszka A, Kunert-Radek J, Pawlikowski M, Stepien H. Serum endostatin levels are elevated and correlate with serum vascular endothelial growth factor levels in patients with pituitary adenomas. Pituitary. 2005; 8:163–168.

4. Komorowski J, Jankewicz J, Stepień H. Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and soluble interleukin-2 receptor (sIL-2R) concentrations in peripheral blood as markers of pituitary tumours. Cytobios. 2000; 101:151–159.
5. Turner HE, Harris AL, Melmed S, Wass JA. Angiogenesis in endocrine tumors. Endocr Rev. 2003; 24:600–632.

6. O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997; 88:277–285.
7. Lee CC, Kano H, Yang HC, et al. Initial Gamma Knife radiosurgery for nonfunctioning pituitary adenomas. J Neurosurg. 2014; 120:647–654.

8. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999; 85:178–187.

9. Vermeulen S, Young R, Li F, et al. A comparison of single fraction radiosurgery tumor control and toxicity in the treatment of basal and nonbasal meningiomas. Stereotact Funct Neurosurg. 1999; 72:Suppl 1. 60–66.

10. Nonoguchi N, Miyatake S, Fukumoto M, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 2011; 105:423–431.

11. Feldman AL, Alexander HR Jr, Bartlett DL, et al. A prospective analysis of plasma endostatin levels in colorectal cancer patients with liver metastases. Ann Surg Oncol. 2001; 8:741–745.

12. Feldman AL, Tamarkin L, Paciotti GF, et al. Serum endostatin levels are elevated and correlate with serum vascular endothelial growth factor levels in patients with stage IV clear cell renal cancer. Clin Cancer Res. 2000; 6:4628–4634.
13. Zucker S, Mirza H, Conner CE, et al. Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer. 1998; 75:780–786.

14. Páez Pereda M, Ledda MF, Goldberg V, et al. High levels of matrix metalloproteinases regulate proliferation and hormone secretion in pituitary cells. J Clin Endocrinol Metab. 2000; 85:263–269.

15. Stepien HM, Kołomecki K, Pasieka Z, Komorowski J, Stepień T, Kuzdak K. Angiogenesis of endocrine gland tumours--new molecular targets in diagnostics and therapy. Eur J Endocrinol. 2002; 146:143–151.

16. O'Sullivan EP, Woods C, Glynn N, et al. The natural history of surgically treated but radiotherapy-naïve nonfunctioning pituitary adenomas. Clin Endocrinol (Oxf). 2009; 71:709–714.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download