Abstract
A postoperative epidural hematoma (EDH) is a serious and embarrassing complication, which usually occurs at the site of operation after intracranial surgery. However, remote EDH is relatively rare. We report three cases of remote EDH after brain tumor surgery. All three cases seemed to have different causes of remote postoperative EDH; however, all patients were managed promptly and showed excellent outcomes. Although the exact mechanism of remote postoperative EDH is unknown, surgeons should be cautious of the speed of lowering intracranial pressure and implement basic procedures to prevent this hazardous complication of brain tumor surgery.
A postoperative intracranial hematoma is one of the most serious complications of cranial surgery. Specifically, an epidural hematoma (EDH) is an embarrassing complication that can occur after intracranial surgery. The reported incidence of postoperative EDHs was approximately 1.0% [12]. An EDH can develop regionally; adjacently; or distantly, in a remote area that is removed from the operation site [1]. There are some reports of remote EDHs that occurred after a ventriculo-peritoneal shunt operation [34567891011121314] or a decompressive craniectomy [1516]. Few cases of postoperative remote EDHs after brain tumor surgery have been reported [217181920212223], especially in posterior fossa surgery [24252627]. The exact mechanism of remote postoperative EDH development is unclear; however, several hypotheses have been proposed. Most often, a sudden drop of intracranial pressure (ICP), developed by the excessive loss of a substantial volume of cerebrospinal fluid (CSF) during surgery, is pointed to as a significant cause of remote EDH.
In the present report, we describe three patients who developed a remote EDH after brain tumor surgery. In addition, we review the literature of remote postoperative EDHs after brain tumor surgery, discuss their precise developmental mechanisms, and suggest methods for preventing them.
A 26-year-old male patient had a 6-month history of headaches. His initial brain computed tomography (CT) scan showed a 4.6×3.5 cm sized mass in the left cerebellopontine angle. This lobulated contoured mass showed heterogenous en-hancement, with internal cystic portions on T1-weighted images of magnetic resonance imaging (MRI) scans (Fig. 1A). Hydrocephalus was not definite. In preoperative hematological studies, no coagulopathy was detected. The patient underwent surgery to remove the tumor via a retrosigmoid approach. After opening the dura, the cerebellum was tense and bulging, the cerebellomedullary cistern was opened, and CSF release was completed at a moderate speed to prevent the rapid reduction of ICP. A complete microsurgical resection of the tumor was achieved in a piecemeal fashion.
After the surgery a postoperative CT scan was performed immediately, as per routine. The CT scan revealed EDHs in both parietal areas, which were remote from the site of the craniotomy (Fig. 1B). However, the patient awoke from general anesthesia well and had no neurological deficits. After 2 weeks postoperatively, without any procedure for the EDH, a follow-up CT scan was performed and it showed no changes in their size (Fig. 1C). The density of the EDH changed as a chronic hematoma. The patient was discharged without any neurological deficits. Postoperative MR images showed that the tumor mass was totally removed (Fig. 1D). The tumor was histologically diagnosed as an acoustic schwannoma.
A 12-year-old male patient had a 9-month history of polydipsia and a 1-month history of visual disturbances. A brain MRI was performed and it showed a 2.0×2.0 cm sized heterogenous mass with enhancement in the suprasellar area (Fig. 2A). On the basis of age and the neuroimaging findings, the initial suspected diagnosis was craniopharyngioma. No hydrocephalus was observed and no coagulopathy was detected in preoperative studies. The patient underwent surgery to remove the tumor via a right pterional approach. Prior to the dura opening, dural tag-up sutures were made along the margin of the craniotomy. There was no excessive or rapid CSF drainage during surgery. After the gross total removal of the tumor, the brain was remarkably sunken down. A complete microsurgical resection of the tumor was achieved in a piecemeal fashion.
After the surgery a postoperative CT scan was performed immediately, as per routine. The CT scan showed no specific complications, such as intracranial hemorrhaging (Fig. 2B). However, after two days postoperatively the patient had symptoms of nausea and a severe headache. A CT scan was performed and it showed an EDH in the right parietal area, which was remote from the site of the craniotomy (Fig. 2C). As the patient was tolerant, we did not perform any immediate procedure for this EDH. After 20 days postoperatively, the patient complained of an aggravated headache. A CT scan was performed and the density of the EDH showed resolution of the hemorrhage. However, the amount of EDH was enlarged (Fig. 2D). Burr hole trephination was conducted and EDH was completely evacuated (Fig. 2E). The patient was discharged without any neurological deficits. Postoperative MR images showed that the tumor mass was totally removed (Fig. 2F). The tumor was histologically diagnosed as a craniopharyngioma.
A 15-year-old female patient had 3-month history of gait disturbance and mild dysarthria. A brain MRI was done and it showed a 7.8×5.8 cm sized, dumbell shaped huge mass involving left cerebellopontine angle; middle cranial fossa; and Meckel's cave which was compressing the midbrain, pons, and cerebellum (Fig. 3A). The mass was well demarcated and showed heterogenous enhancement. Based on these neuroimaging findings, the diagnosis of trigeminal schwannoma was suspected. Ventricular enlargement was noted and no coagulopathy was detected in preoperative hematologic studies. In the operation room, we firstly performed extraventricular drainage (EVD). After performing EVD, we used a left anterior petrosal approach with the patient in the supine position. Before opening the dura, dural tag-up sutures were made along the margin of the craniotomy. Anterior petrosectomy was done extradurally by retracting the frontal lobe. The EVD catheter was mostly closed during surgery. After dural opening, brain edema was severe and the EVD catheter was intermittently opened. However, CSF was not drained excessively or rapidly during surgery. After the subtotal removal of the tumor, the brain seemed to be tense and bulging. We planned a staged operation for the remaining tumor and finished the surgery.
Postoperatively, she was reversed from anesthesia but remained in a Glasgow Coma Scale of E1VtM3. Her pupils were dilated and not reacting. An emergent CT scan was immediately obtained, which showed a huge EDH that was compressing both frontal lobes (Fig. 3B). Coagulation parameters were within normal limits. She underwent an emergency craniotomy and an evacuation of the EDH. After opening the previous bone flap, some leaks were observed between the margin of the skull bone and the dura, which were tagged up by suturing. An extended craniotomy was performed and the EDH was completely removed. After removing the EDH, she awoke from general anesthesia well but showed a slightly drowsy mentality. The EDH was totally evacuated in a postoperative CT scan (Fig. 3C).
One day after initial surgery, a second operation for the tumor was performed. We used a retrosigmoid approach in the lateral park-bench position. The remaining tumor was completely removed in a piecemeal fashion. After the surgery, a CT scan was performed immediately and it showed no specific complications. The patient was discharged without any neurological deficits. Postoperative MRI images showed that the tumor gross was totally removed (Fig. 3D). The tumor was histologically diagnosed as a schwannoma.
A postoperative EDH is a well-known serious complication of intracranial surgery. It usually occurs at the site of the operation. A remote EDH can occur distant to the site of the craniotomy, however, it is quite rare and to our knowledge only a few cases have been reported in the literature (Table 1). These hematomas may be ipsilateral, contralateral, or bilateral, including multiple locations. Various hypotheses regarding the pathophysiology of this remote EDH have been suggested [3151628]. These hypotheses include a sudden decrease in ICP; massive drainage of CSF; unequal distribution of ICP, which causes brain shifting; underlying coagulopathies; and excessively powerful pin fixation, which penetrates the inner table of the skull bone. The major cause of remote EDH seems to be the excessive loss of CSF during surgery, which may cause brain shifting and create negative pressure at a remote area. In many reported cases of remote EDHs, the neurosurgical procedures involved opening the ventricular system or CSF cisterns, which caused the loss of a substantial volume of CSF during surgery [29]. Most authors have suggested that a sudden reduction in ICP may cause traction on the meningeal vessels, such that the negative pressure strips the dura from the inner table of the skull, causing the extradural vessel to bleed. This could cause further dural detachment with more hematoma expansion [19242527]. Other reports have described cases of postoperative remote EDHs that might have resulted from coagulopathy or the use of pins for rigid fixation during surgery. Additionally, as many patients with remote EDHs tend to be young, age could be another trigger factor (Table 1). The adhesion between the dura and the inner table of the skull bone grows stronger with age and the elasticity of the dura is better at young ages; this could result in easier separation of dura from constant negative pressure. However, no single perioperative factor can reliably predict the occurrence of remote site hemorrhages (Table 1).
In our cases, excessive drainage of CSF could be one cause of remote EDH. It could also be caused by opening the CSF cisterns (case 1 and 2, especially in case 1) or by EVD (case 3). However, to prevent remote EDH in case 2, we paid attention to the release of CSF and ensured it was neither rapid nor excessive. Moreover, in case 3 the EVD catheter was mostly closed during surgery. It was intermittently opened only to control severe brain edema. In our opinion, CSF was not drained excessively or rapidly during surgery in cases 2 and 3. Therefore, over-drainage during surgery could only be a cause of remote EDH in case 1. Furthermore, the patients' coagulation profiles were all within the normal range and the location of the EDH was not near the site of pin fixation.
In case 2, the EDH was not seen in the postoperative CT scan, which was performed immediately after surgery. However, pneumocephalus was noted in both frontal areas (Fig. 2B). We think that the expansion of the brain toward the empty space of pneumocephalus caused the negative pressure, and caused a delayed remote EDH in the contralateral side of the pneumocephalus.
In case 3, something might have happened during surgery when the brain edema was aggravated. Since the EVD catheter was mostly closed during the initial surgery and we found some bleeding between the margin of the skull and the dura of the previous craniotomy site during the surgery for EDH, the loosening of tag-up suture seems to be the major cause of the remote EDH. We think some tag-up sutures were slackened during the initial surgery, due to the extradural retraction for the anterior petrosal approach. In addition, the bleedings during surgery might have fallen into the loosened space, causing more dural detachment and further hematoma expansion to the dependent portion (both frontal areas) in the supine position. Of course age could also be a trigger factor in all 3 cases.
Therefore we believe that, especially in young patients, a sudden lowering of ICP by excessive or rapid CSF drainage should be avoided. Moreover, basic procedures, such as filling the normal saline in the intradural space before the last dural suture to prevent pneumocephalus or performing dural tag-up sutures tightly along the margin of the craniotomy should always be remembered. It is also important to detect remote EDHs early by immediate postoperative brain CT scans, and to manage and adequately prevent hazardous complications while achieving an excellent prognosis.
In conclusion, here, we report 3 cases of brain tumor surgeries that were complicated by remote postoperative EDHs. Gradual reduction of ICP and paying particular attention to basic procedures may prevent this hazardous complication in brain tumor surgery, especially in young patients. Furthermore, a high index of suspicion, a prompt diagnosis, and emergent management is important to achieve an excellent prognosis.
Figures and Tables
Fig. 1
Case 1. A: Preoperative gadolinum-enhanced T1-weighted MRI shows 4.6×3.5 cm sized heterogenously enhancing mass located in the cerebellopontine angle. B: Immediate postoperative brain CT scan shows acute EDHs in bilateral parietal regions (arrows). C: Without any procedure for EDHs, 2 week follow-up CT scan shows no change in the size of EDHs and the density of EDHs has changed. D: Postoperative gadolinum-enhanced T1-weighted MRI shows the tumor gross totally removed. EDH, epidural hematoma.
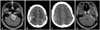
Fig. 2
Case 2. A: Preoperative gadolinum-enhanced T1-weighted MRI shows 2.0×2.0 cm sized heterogenously enhancing mass located in suprasellar area. B: Immediate postoperative brain CT scan shows no specific complications. C: CT scan in 2 postoperative days shows EDH in right parietal area (arrow). D: After 2 weeks follow-up, EDH was resoluted but enlarged. E: After burr hole trephination, EDH was completely evacuated. F: Postoperative gadolinum-enhanced T1-weighted MRI shows the tumor gross totally removed. EDH, epidural hematoma.
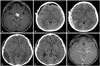
Fig. 3
Case 3. A: Preoperative gadolinum-enhanced T1-weighted MRI shows 7.8×5.8 cm sized dumbell shaped huge mass involving left cerebellopontine angle, middle cranial fossa and Meckel's cave. B: Immediate postoperative brain CT scan shows acute EDHs (arrow) in bilateral frontal area. C: After craniotomy, EDH was completely evacuated. D: Postoperative gadolinum-enhanced T1-weighted MRI shows the tumor gross totally removed. EDH, epidural hematoma.
Table 1
Reported cases of remote postoperative EDH after brain tumor surgery

| Author (yr) | Sex/age | Diagnosis | Location of tumor | Size of tumor | EVD/VP shunt | Approach or craniotomy site | Location of EDH | Surgery for EDH | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| Lourie and Young (1974) [17] | 33/F | Meningioma (olfactory groove) | F (both) | N/A | None | Subfrontal | O (both) | Craniectomy | Death |
| Sinar and Lindsay (1986) [18] | |||||||||
| Case 1 | 32/M | Meningioma (olfactory groove) | F (right) | N/A | None | Subfrontal | P-O (right) | Craniotomy | Cure |
| Case 2 | 24/M | Meningioma (convexity) | F (left) | N/A | None | F | P (left) | Burr hole trephination | Cure |
| Kalfas and Little (1988) [2] | |||||||||
| Case 1 (of 7 cases) | 17/M | Glioblastoma | Pineal region | N/A | None | Supracerebellar infratentorial | O (left) & P (right) | N/A | N/A |
| Bae et al. (2001) [22] | |||||||||
| Case 1 | 27/M | Ependymoastrocytoma | Lateral ventricle (left) | N/A | EVD | F-T | F (right) | Craniotomy | Cure |
| Case 2 | 35/M | Craniopharyngioma | T (left) | N/A | None | T | T-P (right) | Craniotomy | Cure |
| Case 3 | 27/M | Mixed germ cell tumor | Pineal region | N/A | VP shunt | Occipital transtentorial | T-P (left) | Craniotomy | Cure |
| Wolfsberger et al. (2004) [24] | 31/F | Choroid plexus papilloma | Fourth ventricle | 4 cm (diameter) | EVD | Suboccipital | F-T-P (left) | Craniotomy | Cure |
| Jeon et al. (2006) [23] | |||||||||
| Case 1 | 19/F | Low-grade glioma | P (right) | N/A | N/A | P | F-T-P (right) | Craniotomy | Cure |
| Case 2 | 34/M | Central neurocytoma | Lateral ventricle (left) | N/A | N/A | F | T-P (left) | Craniotomy | Cure |
| Case 3 | 42/F | Meningioma (convexity) | F (right) | N/A | N/A | F | F-T-O (right) & O (left) | Craniotomy | Cure |
| Case 4 | 61/F | Meningioma (convexity) | T-P (left) | N/A | N/A | F-T-P | P-O (left) | Craniotomy | Cure |
| Case 5 | 45/M | Meningioma (sphenoid wing) | F-T (left) | N/A | N/A | F-T-P | P (left) | Craniotomy | Cure |
| Pandey et al. (2008) [25] | 5/F | Medulloblastoma | Midline posterior fossa | N/A | EVD | Suboccipital | F (both) | Craniotomy | Cure |
| Borkar et al. (2009) [19] | 18/M | Ganglioglioma | T (left) | N/A | None | T | F (right) | Craniotomy | Cure |
| Avci et al. (2010) [26] | 9/F | Dermoid cyst | Midline posterior fossa | 6×6 cm | EVD | Suboccipital | T-P (left) | Craniotomy | Cure |
| Lim et al. (2010) [27] | 9/M | Mature teratoma | Pineal region | N/A | EVD | Supracerebellar infratentorial | P (both) | Craniotomy | Cure |
| Jin et al. (2013) [20] | |||||||||
| Case 1 | 20/M | Central neurocytoma | Lateral ventricle (left) | N/A | N/A | F | N/A | N/A | Cure |
| Case 2 | 47/F | Central neurocytoma | Lateral ventricle (left) | N/A | N/A | F | N/A | N/A | Cure |
| Cui et al. (2013) [21] | 45/F | Central neurocytoma | Lateral ventricle (left) | 4.0×2.5 cm | EVD | F | P-O (left) | Craniotomy | Cure |
| Present report (2015) | |||||||||
| Case 1 | 26/M | Acoustic schwannoma | CPA (left) | 4.6×3.5 cm | None | Retrosigmoid | P (both) | Observation | Cure |
| Case 2 | 12/M | Mature teratoma | Suprasellar | 2.0×2.0 cm | None | Pterional | P (right) | Burr hole trephination | Cure |
| Case 3 | 15/F | Trigeminal schwannoma | CPA (left), middle | 7.8×5.8 cm | EVD | Anterior petrosal | F (both) | Craniotomy | Cure |
References
1. Fukamachi A, Koizumi H, Nagaseki Y, Nukui H. Postoperative extradural hematomas: computed tomographic survey of 1105 intracranial operations. Neurosurgery. 1986; 19:589–593.

2. Kalfas IH, Little JR. Postoperative hemorrhage: a survey of 4992 intracranial procedures. Neurosurgery. 1988; 23:343–347.

3. Byrappa V, Redhu S, Varadarajan B. Delayed incidental diagnosis of postoperative extradural hematoma following ventriculoperitoneal shunt. J Neurosci Rural Pract. 2015; 6:94–96.

4. Noleto G, Neville IS, Tavares WM, et al. Giant acute epidural hematoma after ventriculoperitoneal shunt: a case report and literature review. Int J Clin Exp Med. 2014; 7:2355–2359.
5. Louzada PR, Requejo PR, Barroso MV, et al. Bilateral extradural haematoma after acute ventricular over-drainage. Brain Inj. 2012; 26:95–100.

6. Chauvet D, Sichez JP, Boch AL. [Early epidural hematoma after CSF shunt for obstructive hydrocephalus]. Neurochirurgie. 2009; 55:350–353.
7. Lee SC, Lee ST, Lui TN. Epidural hematoma of the cervical spine after cervical laminectomy in a patient with ventriculo-peritoneal shunt. J Clin Neurosci. 2004; 11:302–304.

8. Hamlat A, Heckly A, Doumbouya N, Seigneuret E, Brassier G. Epidural hematoma as a complication of endoscopic biopsy and shunt placement in a patient harboring a third ventricle tumor. Pediatr Neurosurg. 2004; 40:245–248.

9. Alsheheri MA, Binitie OP. Acute epidural hematoma following restoration of ventriculoperitoneal shunt patency. Neurosciences (Riyadh). 2004; 9:312–314.
10. Power D, Ali-Khan F, Drage M. Contralateral extradural haematoma after insertion of a programmable-valve ventriculoperitoneal shunt. J R Soc Med. 1999; 92:360–361.

11. Harkness W. Contralateral extradural haematoma after ventriculoperitoneal shunt insertion. J R Soc Med. 1999; 92:547.

12. Fujimoto Y, Aguiar PH, Carneiro JD, et al. Spontaneous epidural hematoma following a shunt in an infant with congenital factor X deficiency. Case report and literature review. Neurosurg Rev. 1999; 22:226–229.

13. Pereira CU, Porto MW, de Holanda RR, de Andrade WT. Epidural hematoma after ventriculoperitoneal shunt surgery. Report of two cases. Arq Neuropsiquiatr. 1998; 56:629–632.

14. Baskin DS, Klein MS, Yang WC, Sachdev VP, Malis LI. Traumatic epidural hematoma in shunt dependent patients: a report of two cases. Surg Neurol. 1979; 11:135–139.
15. Huang YH, Lee TC, Lee TH, Yang KY, Liao CC. Remote epidural hemorrhage after unilateral decompressive hemicraniectomy in brain-injured patients. J Neurotrauma. 2013; 30:96–101.

16. Xu GZ, Wang MD, Liu KG, Bai YA. A rare remote epidural hematoma secondary to decompressive craniectomy. J Craniofac Surg. 2014; 25:e17–e19.

17. Lourie H, Young RF. Posterior epidural hematoma following subfrontal tumor removal. Case report. J Neurosurg. 1974; 40:643–646.
18. Sinar EJ, Lindsay KW. Distant extradural haematoma complicating removal of frontal tumours. J Neurol Neurosurg Psychiatry. 1986; 49:442–444.

19. Borkar SA, Sinha S, Sharma BS. Remote site extradural haematoma. J Clin Neurosci. 2009; 16:1097–1098.

20. Jin Y, Qiu Y, Zhang X. Postoperative complications of central neurocytoma. J Craniofac Surg. 2013; 24:e533–e537.

21. Cui Z, Zhong C, Zhang M, et al. Remote epidural haematoma and severe basal ganglia oedema complicating the removal of a central neurocytoma in the lateral ventricle: a case report and lessons learned. Clin Neurol Neurosurg. 2013; 115:365–367.

22. Bae KJ, Kim IM, Yim MB. Remote epidural hematoma following the removal of brain tumors: report of three cases. J Korean Neurosurg Soc. 2001; 30:366–370.
23. Jeon JS, Chang IB, Cho BM, Lee HK, Hong SK, Oh SM. Immediate Postoperative Epidural Hematomas Adjacent to the Craniotomy Site. J Korean Neurosurg Soc. 2006; 39:335–339.
24. Wolfsberger S, Gruber A, Czech T. Multiple supratentorial epidural haematomas after posterior fossa surgery. Neurosurg Rev. 2004; 27:128–132.

25. Pandey P, Madhugiri VS, Sattur MG, Devi BI. Remote supratentorial extradural hematoma following posterior fossa surgery. Childs Nerv Syst. 2008; 24:851–854.

26. Avci E, Dagtekin A, Baysal Z, Karabag H. Intraoperative supratentorial epidural haematoma during removal of a huge posterior fossa dermoid cyst. Neurol Neurochir Pol. 2010; 44:609–613.

27. Lim JW, Yang SH, Lee JS, Song SH. Multiple remote epidural hematomas following pineal gland tumor resection. J Pediatr Neurosci. 2010; 5:79–81.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download