Abstract
lnflammatory pseudotumor (IPT) is a rare, non-neoplastic inflammatory process. It is most commonly occurs in the orbit, but extension into brain parenchyma is uncommon. In a confirmed case of IPT, most cases show good improvement with steroid theraphy. A 50-year-old man with progressive left-eye visual disturbance and mass lesion was admitted in a hospital. A left orbital mass biopsy revealed what was highly suspected as an inflammatory pseudotumor. Steroid pulse therapy with dexamethasone, radiation therapy, and chemotherapy with amphotericin B were performed, but they were not effective in improving the condition of the patient. Revision open surgery was then performed. A follow-up brain enhancement computerized tomography showed an enlarged mass volume and hydrocephalus with periventricular enhancement. As an additional procedure, ventriculoperitoneal shunt and tuberculosis medication were administered. About 2 weeks later, clinical symptoms and radiologic findings improved. We present a case of intra-cranial IPT and discuss further treatment methods.
Inflammatory pseudotumor (IPT) is a rare, non-neoplastic inflammatory process [1]. It is most commonly occurs in the orbit, but extension into brain parenchyma is uncommon [2]. In a confirmed case of IPT, most cases show good improvement with steroid theraphy [2]. We present a case of intra-cranial IPT and discuss further treatment methods.
A 50-year-old man was admitted with progressive left-sided visual disturbance, severe headache, and was in a state of lethargy for one year. The initial modified Rankin Scale (mRS) of the patient was 2 at admission. Painful ophthalmoplegia began in April 2012. A left orbital mass biopsy was performed in another hospital, revealing highly suspected IPT, so he underwent steroid pulse therapy, radiation therapy (RT; 30 Gy/15 fx), and chemotherapy (Cyclophosphamide), though the patient did not respond well to such medical procedures. In April 2013, when he visited our hospital, he has lost his vision and light reflex in the left eye, suggesting that the 3rd, 4th, and 6th nerves were affected. The computerized tomography (CT) scan showed that a spindle shaped, slightly high density mass was noted in the left anterior cavernous sinus and in the orbital apex with pressure erosion of the adjacent bones (Fig. 1A). A magnetic resonance image (MRI) of the brain showed enhanced lesion in the cavernous sinus bilaterally with sphenoid sinus fullness, left pituitary fossa, left orbital apex, a mass in the left temporal lobe measuring about 4.9×4.3×4.3 cm, left distal internal carotid artery, left proximal anterior cerebral artery A1, and left middle cerebral artery M1 segment involvement (Fig. 1B, C). The positron emission tomography-CT scan showed no high metabolism lesion except in the brain (Fig. 1D). Lab findings showed an infective state with elevated white blood cell, erythrocyte sedimentation rate, C-reactive protein in the blood and in the cerebrospinal fluid, but did not detect bacteria or fungi.
We thought it might be an IPT or metastatic cancer, and applied dexamethasone and amphotericin B. Later, craniotomy was done on the left side of the brain to remove a tumor. The pathologic findings presented infiltrating small lymphocytes and uninucleated histiocytes in the fibrous stroma. The lymphoid follicle has surrounding lymphocytes in the hematoxylin and eosin stain. In the keratin finding stain, there were no definite carcinoma with multiple infiltration (Fig. 2). More than 7 days after surgery, the patient slightly recovered left side visual acuity. However, left eye vision and right eye visual acuity decreased at the postoperative 10th day. A follow-up brain enhancement CT showed decreased mass size and enhancement with hydrocephalus (Fig. 3A). An additional procedure, ventriculoperitoneal shunt, was performed. The patient was then administered TB medication 1 month after open craniectomy. About 2 weeks later after TB medication and shunt procedure, blindness in the left eye continued while visual acuity of the right eye improved daily. The follow-up brain CT showed decreased high density lesion at the left anterior cavernous sinus and orbital apex (Fig. 3B, D), and the MRI revealed markedly decreased hyper intensity with strong enhancement lesion (Fig. 3C). The mRS of the patient was 2 upon discharge.
IPT is defined as a localized, solitary, reactive, inflammatory, and non-neoplastic phenomenon occurring in many areas of the body [3]. IPT has been reported in a number of locations, including the lungs, lymph nodes, orbital cavities, and soft tissue [4]. The lungs are apparently the most common site, the liver is the second most common and occurrence in the head and neck is rare, especially central nervous system [2]. Occurrence of this condition in the head and in the neck is rare, especially the central nervous system. IPT of the head and neck can affect the orbit (orbital pseudotumor), orbital apex (orbital apex syndrome), superior orbital fissure (superior orbital fissure syndrome), and anterior, middle, and posterior cavernous sinus (cavernous syndrome) [5]. Other extraorbital sites include nasal cavity, nasopharynx, maxillary sinus, sphenoid sinus, infratemporal fossa, choroid plexus, larynx and trachea, and skull bone. Extension into the brain parenchyma is rarely reported. In a series of 90 consecutive biopsy-proven cases of orbital pseudotumor, eight cases (8.9%) showed radiologic evidence of intracranial spread [2].
The pathogenesis of IPTs remains unclear. Fibrosis and inflammatory cells are present, with irregularly scattered infiltration by lymphocytes, plasma cells, and macrophages, possibly resulting from inflammation [4]. The immune response may indicate an abnormal and prolonged reparative process [6], and it is also caused by infections like Epstein-Barr virus, Cytomegalovirus, and Herpes Simplex Virus [7]. Intracranial IPTs arise mostly from the dural and meningeal structures [4].
Radiographically, the ultrasound showed a variable pattern of echogenicity with ill-defined or well-defined margins. Prominent vascularity may be shown with color or power Dop-pler ultrasound, especially contrast-enhanced power Doppler ultrasound [8]. IPTs are hyperdense, with variable am-ounts of edema on the CT. On the MRI, the T1-weighted ch-aracteristics range from hypointense to hyperintense. Homo-genous enhancement is generally seen after gadolinium ad-ministration. The heterogenous pattern of enhancement may be attributed to foci of calcification within the lesion. On T2-weighted imaging, IPTs are hypointense to hyperintense, and may demonstrate interdigitation with surrounding cortex despite being well encapsulated [9].
The differential diagnosis of our patient's clinical picture include malignant diseases (lymphoma, leukemia, rhabdomyosarcoma, Ewing's sarcoma, and primitive neuroectodermal tumors and lymphomas), sarcoidosis, Wegener's granulomato-sis, vasculitis, tuberculosis, and fungal infections, such as as-pergillosis and mucormycosis [2]. Intracranial inflammatory masses are often associated with the meninges. Thus, they are often misinterpreted as meningiomas with lymphoplasmacytic infiltration [9]. A definite diagnosis of IPT can be made through a biopsy.
The prognosis of intracranial IPTs is poor. This tumor is considered a benign entity, but neoplastic transformation with aggressive growth and additional distant lesions have also been reported [10]. Standard treatment is controversial, but, usually, complete surgical removal is tried at first, or high doses of oral corticosteroids are administered. Most cases improved within 48-72 hours. However, there is no consensus regarding the duration of treatment. We have to consider individualized treatment based on clinical symptoms and radiologic findings. Low-dose RT may be considered for cases in which there is contraindication or a poor response from the use of steroids [2].
In conclusion, IPTs should be considered in the differential diagnosis for any intracranial mass or infectious diseases. A definitive diagnosis cannot be obtained without considering a number of differential diagnoses, requiring further biochemical analysis and tissue sampling for a histological study. Further long-term data are needed to determine the efficacy of the operation, peri-operative radiation, and steroid usage, as well as to define characteristics that predispose patients to progression or recurrence of symptoms.
Figures and Tables
Fig. 1
Neuroimaging findings on admission. A: Pre-operative brain computed tomography (CT). Spindle shaped, slightly high density is noted in the left anterior cavernous sinus and orbital apex. Pressure erosion of the adjacent bones. B and C: Pre-operative brain magnetic resonance image (MRI) T2-weighted image (B). Pre-operative brain MRI T1-weighted enhanced image (C). Mixed intensity lesion at the left pituitary fossa, orbital apex, and middle cranial fossa, with strong enhancement. D: Whole body positron emission tomography-CT. Hypermetabolic, infiltrative lesion at the left anterior skull base, but there is no other definite metastatic lesion.

Fig. 2
Pathological findings. A: Bone marrow fibrosis. B: Bone marrow cell infiltration. Photomicrograph shows infiltrating small lymphocytes and uninucleated histiocytes in the fibrous stroma. Lymphoid follicle with surrounding lymphocytes (hematoxylin and eosin stain; A and B: ×100). C: Keratin negative (no carcinoma). In the keratin finding stain, there are no definitive carcinoma. D: Left coronary artery (LCA) positive (infiltration). LCA positive findings prove there are multiple infiltrations (C and D: ×40).
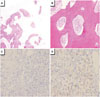
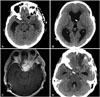
Fig. 3
Neuroimaging findings at post operative periods. A: First post-operative brain computed tomography (CT) (craniotomy with tumor removed). Decreased high density lesion at the left anterior cavernous sinus and orbital apex with hyodrocephalus. B: Second post-operative brain CT (ventriculoperitoneal shunt). Ventriculoperitoneal shunt state. C: Follow-up brain magnetic resonance image T1-weighted enhanced image at post-operative period. Hyper intensity with strong enhancement lesion was markedly decreased. D: Follow-up brain CT at post-operative period 6 months later. Decreased high density lesion with ventricle size.
References
1. Gandhi RH, Li L, Qian J, Kuo YH. Intraventricular inflammatory pseudotumor: report of two cases and review of the literature. Neuropathology. 2011; 31:446–454.

2. Saifudheen K, Jose J, Gafoor VA. Inflammatory pseudotumor of the head presenting with hemiparesis and aphasia. Case Rep Neurol Med. 2011; 2011:176546.

3. Ohue S, Kohno S, Matsui S, Kumon Y, Ohnishi T. Inflammatory pseudotumor in the lateral ventricle. Case report. Neurol Med Chir (Tokyo). 2012; 52:599–602.
4. Lin YJ, Yang TM, Lin JW, Song MZ, Lee TC. Posterior fossa intracranial inflammatory pseudotumor: a case report and literature review. Surg Neurol. 2009; 72:712–716. discussion 716

5. Lee EJ, Jung SL, Kim BS, et al. MR imaging of orbital inflammatory pseudotumors with extraorbital extension. Korean J Radiol. 2005; 6:82–88.

6. Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey. Semin Diagn Pathol. 1998; 15:85–101.
7. Jung TY, Jung S, Lee MC, et al. Hemorrhagic intracranial inflammatory pseudotumor originating from the trigeminal nerve: a case report. J Neurooncol. 2006; 76:139–142.

8. Park SB, Lee JH, Weon YC. Imaging findings of head and neck inflammatory pseudotumor. AJR Am J Roentgenol. 2009; 193:1180–1186.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download