Abstract
A variety of surgical approaches to temporal horn tumors of the lateral ventricle have been described. Magnetic resonance imaging (MRI) and angiography are the preferred modalities for preoperative evaluation and provide important information for the choice of surgical approach. A 59-year-old man was referred to our hospital due to confusion and gait disturbance. On enhanced MRI, a homogeneous enhanced solitary mass was observed within the temporal horn of the left lateral ventricle with transependymal extension. The lesion was accompanied by increased hypervascular tumor blush on preoperative cerebral angiography. Subtotal removal of the temporal horn tumor was performed because the lesion was identified as lymphoma during surgery. The postoperative course was un-eventful. The patient was referred to the oncology department for conventional chemotherapy. Adjuvant chemotherapy improved the clinical outcome. The pterional-transsylvian approach was beneficial for partial removal of the tumor and tissue diagnosis in this case.
Although several surgical approaches to temporal horn tumors of the lateral ventricle have been introduced, two surgical routes to the temporal horn are commonly performed. These two approaches are the pterional-transsylvian approach and the subtemporal approach through the occipitotemporal sulcus. Neurosurgeons are familiar with the pterional-transsylvian approach but it is a less-established route for accessing temporal horn tumors. It is also a valid alternative for the treatment of intracranial aneurysm and spontaneous intracerebral hemorrhage. Each approach has its advantages and disadvantages. The pterional-transsylvian approach enables safe entry into the temporal horn without injuring the optic radiation and the uncinate fasciculus [1].
The occipitotemporal sulcus approach is also very effective at visual field preservation. However, it does involve a significant degree of retraction and can lead to contusions or injury to the vein of Labbé [2].
From our experience, the transsylvian approach is an appropriate surgical procedure for the diagnosis and management of tumors in the temporal horn.
A 59-year-old man presented with a several-day history of confusion and gait disturbance. His past history included an operation for a cerebellar hemangioblastoma at an outside hospital more than 10 years prior to the current presentation. On examination, the patient was confused and had no cranial nerve deficits. He had an unsteady gait. His motor strength was normal.
MRI revealed a 3 cm, oval-shaped mass in the left temporal horn on T1-weighted sequences, with homogeneous contrast enhancement after gadolinium administration. T2-weighted imaging highlighted edema surrounding the tumor (Fig. 1). Tumor vascularity was assessed preoperatively using cerebral angiography (Fig. 2). Ependymoma and meningioma were considered in the preoperative differential diagnosis. This patient was not a candidate for stereotactic biopsy due to prominent vascular blush of the tumor on preoperative angiography. The patient received preoperative dexamethasone and was loaded with prophylactic valproate anticonvulsant therapy.
At craniotomy, the sylvian fissure was widely split using a combination of sharp and blunt dissection, and the basal cisterns were opened to fully expose the middle cerebral artery. After the inferior circular sulcus of the insula and the limen insula were satisfactorily exposed, a 30-mm incision was made along the inferior circular sulcus of the insula 1 cm posterior to the limen insula. Dissecting the bottom of the inferior circular sulcus of the insula disclosed the underlying tumor around the left temporal horn (Fig. 3). The lesion appeared whitish-gray, soft in consistency, and prone to bleeding. Subtotal removal of the tumor was performed with the use of an ultrasonic surgical aspirator, because an intraoperative frozen biopsy identified lymphoma. The postoperative neurological status of this patient was unchanged, and he was referred to the oncology department for further evaluation (bone marrow aspiration and CT scans) and adjuvant chemotherapy (CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone). The bone marrow biopsy did not detect abnormal marrow cell or infiltration by cancer cells from other parts of the body. Chest and abdomen CT scans did not demonstrate involvement of other organs. An HIV test was negative. Histopathology confirmed a malignant diffuse B-cell non-Hodgkin lymphoma. Adjuvant chemotherapy improved the clinical outcome.
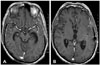
A postoperative MR study was performed 3 months after the surgery and showed complete disappearance of the temporal horn tumor (Fig. 4).
Currently, the temporal horn of the lateral ventricle is usually accessed using the pterional-transsylvian and occipitotemporal sulcus approaches [12]. In a surgical anatomy study, Sincoff et al. [3] categorized three surgical routes according to the direction and surface from which they approach the temporal horn and mesial structures of temporal lobe. They included a transsylvian approach, a lateral transsulcal or transcortical approach through the superior or inferior temporal sulcus and their adjacent gyri, and a subtemporal approach through the parahippocampal or fusiform gyri.
For lesions in the temporal horn of the lateral ventricle, any approach is justifiable if the risks of morbidity and mortality associated with treatment are not greater than another approach. Morbidity associated with surgical routes to the temporal horn consists mainly of visual field deficits and aphasia. Injury to the optic radiation and the uncinate fasciculus are closely related to visual field deficits and aphasia.
The optic radiation is a bundle of fibers that extends from the lateral geniculate body to the visual area in the occipital lobes. According to the direction of its fibers, the optic radiation may be divided into anterior, middle, and posterior parts.
The anterior loop of the optic radiation is called Meyer's loop, and its anterior extension is variable, ranging from 10 mm in front to 5 mm behind the tip of the temporal horn (average 5±3.9 mm in front) [2]. The anterior part of the optic radiation represents the upper quadrants of the visual field. Coppens et al. [4] have also described that the optic radiation fibers completely cover the superior and lateral walls of the temporal horn and the lateral portion of the tip of the temporal horn. The entire medial wall of the temporal horn is free of optic radiation fibers, except at the level where these fibers arise from the lateral geniculate body.
The uncinate fasciculus is a bidirectional, long-range white matter tract beneath the limen insula that connects the medial and lateral orbitofrontal cortex with the anterior temporal lobes. Although abnormalities in the uncinate fasciculus have been associated with several psychiatric disorders, episodic memory, and language and social emotional processing, its exact function is not well understood. Papagno et al. [5] assessed patients with removal of the left uncinate fasciculus due to surgery for low-grade gliomas and found significant deficits in naming famous faces immediately after surgery and 3 months later.
In a retrospective study by D'Angelo et al. [6], the surgical approach to lateral ventricle tumors was individualized and based on tumor origin and development: primary or secondary ventricular and associated transependymal development.
Various types of tumors occur in the temporal horn of the lateral ventricle. Ependymoma, subependymoma, central neurocytoma, low-grade glioma, high-grade glioma, choroid plexus papilloma, meningioma, and epidermoid cysts are considered in the differential diagnosis before surgery [2]. The incidence of intraventricular lymphoma remains unknown. Intraventricular lymphoma is mainly found in the body of the lateral ventricle [7]. In a clinical MRI study of central nervous system (CNS) lymphoma, Küker et al. [8] reported that intraventricular lymphoma, visualized as a mass lesion originating from the ventricular borders protruding into the internal spaces, was found in 9 of 100 patients. These exophytic tumors were considered to extend into the ventricular lumen. CNS lymphoma with intraventricular lesions may be a distinct subtype of cerebral lymphoma. Therefore, preoperative MRI cannot distinguish intraventricular solitary lymphoma from other primary intraventricular neoplasms and does not add to the specificity of the differential diagnosis.
Consequently, tissue diagnosis was required for our patient. We preferred an open surgical resection and biopsy to stereotactic needle biopsy, because the patient had an intraventricular lesion with transependymal extension and was at risk for hemorrhage. We chose the pterional-transsylvian approach in order to avoid postoperative visual field deficits and aphasia.
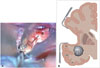
For the pterional-transsylvian approach, the inferior circular sulcus of the insula and the limen insula are important landmarks for entering the temporal horn. The insular circular sulcus is a deep furrow and one of three circular parts (superior, anterior, and inferior parts). The superior, inferior, and anterior sulcus encircles the insula and separates the insula from the frontoparietal and temporal opercula. Intraoperative images show the inferior insular sulcus, indicated with white dotted lines (Fig. 3A). Under normal conditions, the temporal horn can be reached by dissecting the bottom of the inferior circular sulcus of the insula, following its direction 1 cm posterior to the limen insula (Fig. 3B). The more posterior the dissecting site in the inferior circular sulcus, the easier the entry into the temporal horn. After dissecting the bottom of the inferior circular sulcus of the insula, we easily identified the tumor in our case.
In conclusion, accurate diagnosis of a temporal horn tumor is difficult using only imaging findings. It is also difficult to access a temporal horn tumor using only stereotactic biopsy. Neurosurgeons are familiar with the pterional-transsylvian approach in neurosurgical disease. In our experience, this approach is very helpful for diagnosing and removing tumors in the temporal horn without risk of injury to the optic radiation and uncinate fasciculus.
Figures and Tables
Fig. 1
Preoperative nonenhanced axial (A), coronal (B), and gadolinium-enhanced axial (C and D) MRI scans demonstrate a large tumor of ovoid shape inside the left temporal horn with homogenous enhancement and peritumoral edema.
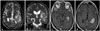
Fig. 3
Intraoperative findings. A: The sylvian fissure is split to identify the inferior insular sulcus. The inferior insular sulcus was mapped with dotted black lines. B: The temporal horn is reached by dissecting the bottom of the inferior circular sulcus of the insula. IL, insula lumen; STG, superior temporal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus.
References
1. Wen HT, Mussi AC, Rhoton AL Jr, de Oliveira E, Tedeschi H. Surgical approaches to lesions located in the lateral, third, and fourth ventricles. In : Sekhar LN, Fessler RG, editors. Atlas of neurosurgical techniques-brain. 1st ed. New York: Thieme Medical Publishers Inc.;2006. p. 507–546.
2. Bertalanffy H, Krayenbuhl N, Wess C, Bozinov O. Ventricular tumors. In : Winn HR, editor. Youmans neurological surgery. 6th ed. Philadelphia: Elsevier;2011. p. 1534–1568.
3. Sincoff EH, Tan Y, Abdulrauf SI. White matter fiber dissection of the optic radiations of the temporal lobe and implications for surgical approaches to the temporal horn. J Neurosurg. 2004; 101:739–746.

4. Coppens JR, Mahaney KB, Abdulrauf SI. An anteromedial approach to the temporal horn to avoid injury to the optic radiation fibers and uncinate fasciculus: anatomical and technical note. Neurosurg Focus. 2005; 18:E3.

5. Papagno C, Miracapillo C, Casarotti A, et al. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011; 134(Pt 2):405–414.

6. D'Angelo VA, Galarza M, Catapano D, Monte V, Bisceglia M, Carosi I. Lateral ventricle tumors: surgical strategies according to tumor origin and development--a series of 72 cases. Neurosurgery. 2005; 56:1 Suppl. 36–45. discussion 36-45




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download