Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare but potentially fatal drug-induced systemic hypersensitivity response characterized by erythematous eruption, fever, leukocytosis with eosinophilia, and internal organ involvement. Antitubercular agents are potential causative agents for DRESS syndrome but difficult to verify as a culprit drug, since antitubercular agents are coadministered as a combination regimen. A 42-year-old female with endobronchial tuberculosis was diagnosed with DRESS syndrome after 4-week treatment of isoniazid, rifampicin, ethambutol, and pyrazinamide with prednisolone 50 mg. All the antitubercular agents were stopped and replaced with levofloxacin, cycloserine, p-aminosalicylic acid, and kanamycin. However, severe exacerbation of DRESS syndrome compelled the patient to discontinue the administration of the second-line anti-tubercular agents. Two months later, the patient underwent a patch test for all the antitubercular agents which had been used, and the results showed positivity to isoniazid and cycloserine. We report a rare case of DRESS syndrome that reacted to cycloserine as well as isoniazid. Development of coreactivity to other drugs should be differentiated with a flare-up reaction in the management of DRESS syndrome.
REFERENCES
1. Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011; 124:588–97.

2. Nam YH, Park MR, Nam HJ, Lee SK, Kim KH, Roh MS, et al. Drug reaction with eosinophilia and systemic symptoms syndrome is not uncommon and shows better clinical outcome than generally recognised. Allergol Immunopathol (Madr). 2015; 43:19–24.

3. Ye YM, Hur GY, Kim SH, Ban GY, Jee YK, Naisbitt DJ, et al. Drug-specific CD4+ T-cell immune responses are responsible for antituberculosis drug-induced maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms syndrome. Br J Dermatol 2016 Jul 4 [Epub].https://doi.org/10.1111/bjd.14839.
4. Choi JH, Heo NY, Park SH, Park CS, Jo KM, Kim WG, et al. Concomitant drug reaction with eosinophilia and systemic symptom syndrome from ethambutol and autoimmune hepatitis from isoniazid. Korean J Gastroenterol. 2016; 67:267–71.

5. Arruti N, Villarreal O, Bernedo N, Audicana MT, Velasco M, Uriel O, et al. Positive Allergy Study (Intradermal, Patch, and Lymphocyte Transfor-mation Tests) in a Case of Isoniazid-Induced DRESS. J Investig Allergol Clin Immunol. 2016; 26:119–20.

6. Shin KS, Park MS. Eosinophilic polymyositis and DRESS (drug rash with eosinophilia and systemic symptoms) syndrome by antitubercular agents. J Korean Neurol Assoc. 2016; 34:154–6.

7. Zhang SN, He QX, Yang NB, Ni SL, Lu MQ. Isoniazid-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome presenting as acute eosinophilic myocarditis. Intern Med. 2015; 54:1227–30.

8. Toujani S, Zaiem A, Mjid M, Ouahchi Y, Ben Salah N, Louzir B, et al. Drug rash with eosinophilia and systemic symptoms to antituberculosis treatment. Tunis Med. 2015; 93:590–3.
9. Moon SD, Won HK, Cho JY, Kang MK, Kim JY, Park HK, et al. Successful readministration of second-line antituberculous agents in a patient with near-fatal drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Allergy Asthma Respir Dis. 2015; 3:297–301.

10. Lee JY, Seol YJ, Shin DW, Kim DY, Chun HW, Kim BY, et al. A case of the drug reaction with eosinophilia and systemic symptom (DRESS) following isoniazid treatment. Tuberc Respir Dis (Seoul). 2015; 78:27–30.

11. Thong BY, Chia FL, Tan SC, Tan TC, Leong KP, Tan JW, et al. A retrospective study on sequential desensitization-rechallenge for antituberculosis drug allergy. Asia Pac Allergy. 2014; 4:156–63.

12. Shebe K, Ngwanya MR, Gantsho N, Lehloenya RJ. Severe recurrence of drug rash with eosinophilia and systemic symptoms syndrome secondary to rifampicin patch testing in a human immunodeficiency virus-in-fected man. Contact Dermatitis. 2014; 70:125–7.

13. Palmero D, Castagnino J, Musella RM, Mosca C, González Montaner P, de Casado GC. Difficult clinical management of anti-tuberculosis DRESS syndrome. Int J Tuberc Lung Dis. 2013; 17:76–8.
14. Kim JY, Sohn KH, Song WJ, Kang HR. A case of drug reaction with eosinophilia and systemic symptoms induced by ethambutol with early features resembling Stevens-Johnson syndrome. Acta Derm Venereol. 2013; 93:753–4.

15. Kim JH, Jang SH, Kim DH, Park S, Kim DG, Jung KS. A case of DRESS syndrome induced by the antituberculosis drugs, prothionamide, and para-aminosalycilic acid. Ann Allergy Asthma Immunol. 2013; 110:118–9.

16. Draz N, Datta S, Webster DP, Cropley I. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome secondary to antituberculosis drugs and associated with human herpes virus-7 (HHV-7). BMJ Case Rep 2013 Jul 31. 2013. https://doi.org/10.1136/bcr-2013-010348.
17. Cheng J, Rawal S, Roberts A, Guttman OR. Drug reaction with eosinophilia and systemic symptoms syndrome associated with antituberculosis medications. Pediatr Infect Dis J. 2013; 32:1388–90.

18. Rodríguez R, Jover V, Orozco I, Domenech J. DRESS syndrome in a 19-year-old patient following the administration of first-line antituberculosis drugs. J Investig Allergol Clin Immunol. 2012; 22:380–1.
19. Jung ES, Choi B, Choi HS, Kim BH, Ha M, Shin D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome induced by ethambutol and rifampin. Infect Chemother. 2012; 44:197–200.

20. Lee SW, Yoon NB, Park SM, Lee SM, Um SJ, Lee SK, et al. Antituberculosis drug-induced drug rash with eosinophilia and systemic symptoms syndrome confirmed by patch testing. J Investig Allergol Clin Immunol. 2010; 20:631–2.
21. Yoon HJ, Lee DH, Sin WS, Suh DH. Two cases of antituberculosis drug-induced hypersensitivity syndrome. Korean J Dermatol. 2007; 45:635–9.
22. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed.Cheongju: Korea Centers for Disease Control and Prevention;2014.
Fig. 1.
Summary of clinical course. Clinical course of the patient from initial administration of first-line antitubercular medication (day 0). H, isoniazid; R, rifampin; Z, pyrazinamide; E, ethambutol; Lz, linezolid; SMX-TMP, sulfamethoxazole-trimethoprim; Lev, levofloxacin; CS, cycloserine; PAS, p-aminosalicylic acid; KM, kanamycin; DRESS, drug reaction with eosinophilia and systemic symptoms; ALT, alanine aminotransferase.
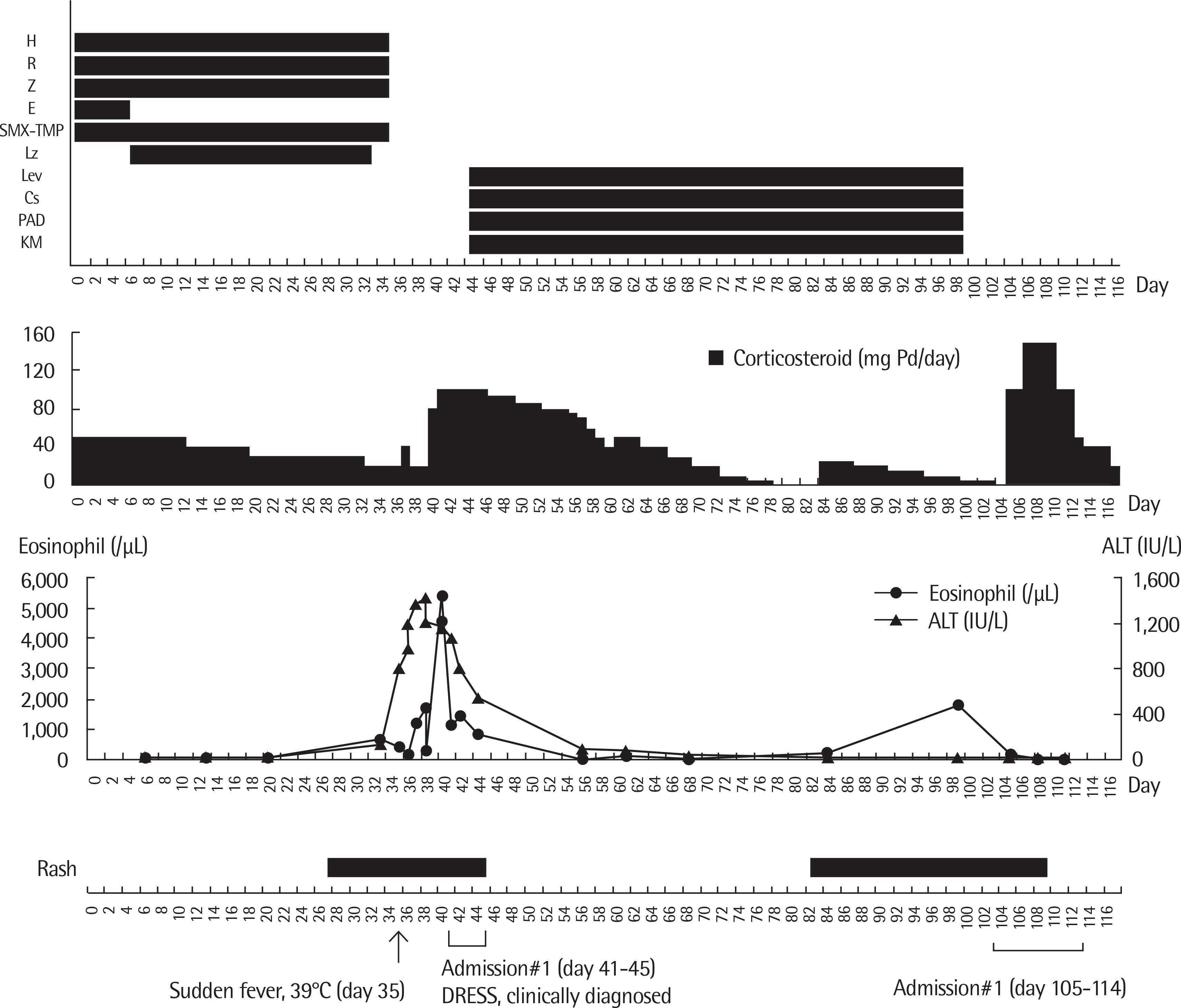
Fig. 2.
Skin finding of patch test to antitubercular agents. Read at 48 hours (A), and 72 hours (B). Test drugs are isoniazid (INH), rifampin (R), pyridoxine (P), sul-famethoxazole-trimethoprim (sep), p-aminosalicylic acid (PAS), kanamycin (K), linezolid (zyv), cycloserine (CS), levofloxacin (LV), pyrazinamide (PZA), ethambutol (E), vaseline (V) in clockwise direction from the right top.
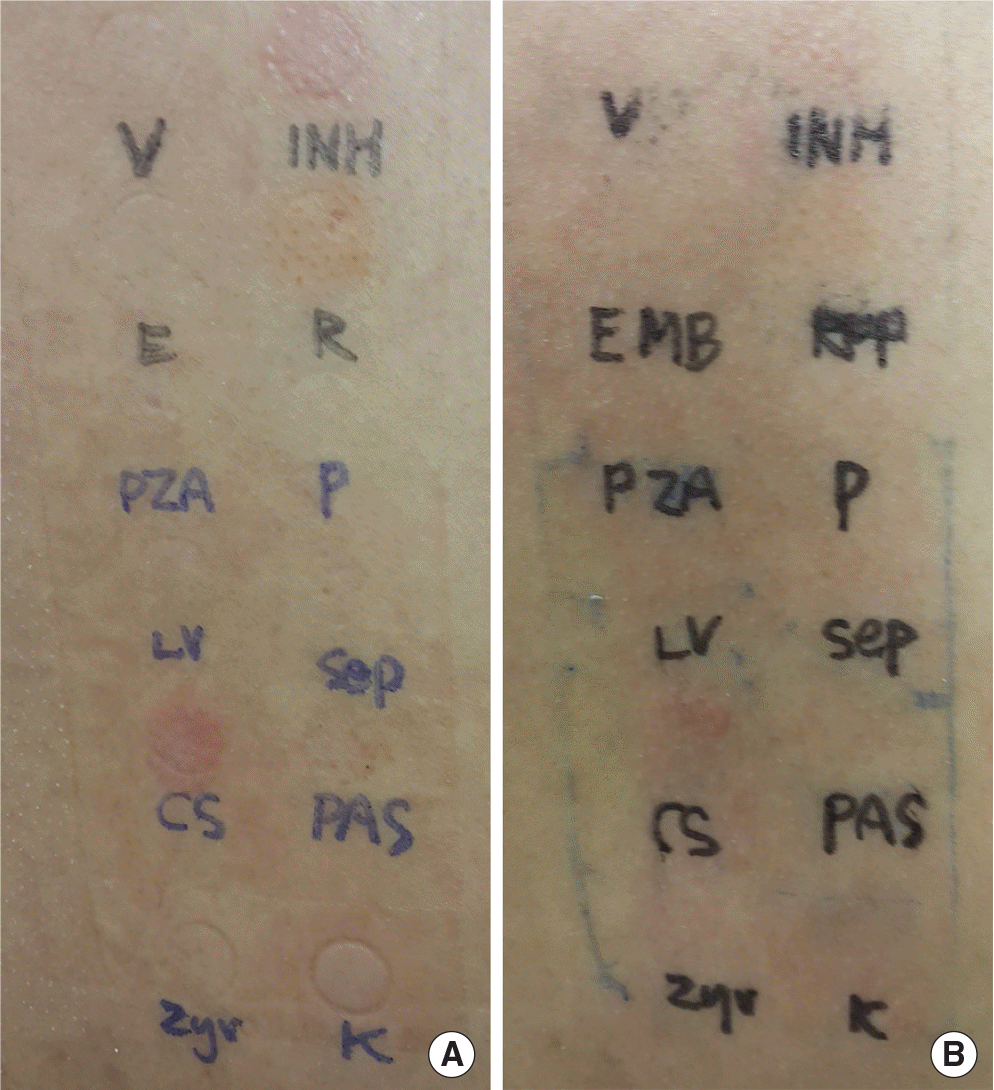
Table 1.
Clinical features and implicated drugs of 38 cases with of antitubercular medication-related DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome
| Study | Age/sex | Types of tuberculosis | Time to onset | Implicated drugs |
|---|---|---|---|---|
| Ye et al., 20163 | 61/M | N/A | 30 Days | HREZ |
| 56/F | N/A | 30 Days | HR | |
| 35/F | N/A | 12 Days | H | |
| 43/F | N/A | 60 Days | HREZ | |
| Choi et al., 20164 | 22/F | Lung | 2 Months | E |
| Arruti et al., 20165 | 21/M | Lung | 1 Months | H |
| Shin et al., 20166 | 17/F | Pleura | 1 Months | HREZ |
| Zhang et al., 20157 | 43/M | Meninx | 2 Months | H |
| Toujani et al., 20158 | 45/F | Lymph node | 34 Days | HREZ |
| Moon et al., 20159 | 53/M | Lung (MDR) | 20 Days | PTH, PAS, S |
| Lee et al., 201510 | 71/M | Meninx | 2 Months | H |
| Thong et al., 201411 | 74/M | Lung | 12 Days | HRZ |
| 34/M | Lung | 5 Days | HRES | |
| 50/M | Lung | N/A | HREZ | |
| 30/M | Lymph node | 11 Days | HREZ | |
| 47/M | Lung | 72 Days | HRE | |
| 62/M | Lung | 80 Days | HRE | |
| Shebe et al., 201412 | 34/M | N/A | 4 Weeks | R |
| Palmero et al., 201313 | 27/F† | Lung, peritoneum | 30 Days | HREZ |
| 31/F | Pleura | 21 Days | R | |
| 32/F | Lung | 30 Days | R | |
| 23/M | Lung | 40 Days | H | |
| 30/F | Lung, ganglion | 35 Days | HE | |
| 35/M | Lung, pleura | 30 Days | HREZ | |
| 31/M | Lung (not cavitated) | 12 Days | HRE | |
| 41/M | Lung, larynx | 60 Days | HRZ | |
| 38/M | Lung (miliary) | 21 Days | R | |
| 18/F | Lung (MDR) | 30 Days | Z, Mfx | |
| 34/F | Lung (MDR) | 60 Days | CS, Eth, Mfx, Lev, PAS | |
| Kim et al., 201314 | 68/F | Pericardium | 7 Weeks | E |
| Kim et al., 201315 | 33/M | Lung (MDR) | 3 Weeks | PTH, PAS |
| Draz et al., 201316 | 41/F | Lymph node | 21 Days | HREZ |
| Cheng et al., 201317 | 12/M | Lung | 4 Weeks | HEZ |
| Rodriguez et al., 201218 | 19/M | Lymph node | 2 Weeks | HREZ |
| Jung et al., 201219 | 26/F | Lung | 4 Weeks | RE |
| Lee et al., 201020 | 72/F | Urethra | 4 Weeks | HRE |
| Yoon et al., 200721 | 32/M | Lung | 2 Weeks | HREZ |
| 25/F | Lung | 4 Weeks | HREZ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download