Abstract
Purpose
Recently, component-resolved diagnosis (CRD) using microarray technology has been introduced to the field of clinical allergy. This study was aimed to investigate the clinical usefulness of microarray-based IgE detection for diagnosing clinical raw fruit allergy in birch pollen-sensitized children.
Methods
Thirty-one children with allergic disease who had been sensitized to pollen were studied. A pollen-sensitized patient was defined as having an allergen-specific history with concomitant positive skin-prick tests (SPTs) to natural allergen extracts or positive allergen-specific IgE. All subjects underwent SPTs for pollen and fruit. In all subjects, specific IgE to pollen and fruit were measured by ImmunoCAP. Specific IgE antibodies to allergen components were determined by a customized allergen microarray (ISAC).
Results
Thirteen of the 31 patients (41.9%) had a history of fruit hypersensitivity with positive SPTs. Measuring IgE to allergen components by ISAC, all the 13 patients with fruit hypersensitivity were positive to at least one of Mal d 1, Pru p 1, Pru p 3, Act d 8, and Act d 2 compared to 12 of the 13 patients (92.3%) who had at least 1 positive IgE to fruits (apple, peach, and kiwi) using ImmunoCAP. The sensitivity of ISAC microarray was 100.0% for the diagnosis of fruit hypersensitivity, but its specificity was 27.7% (5/18). The sensitivity of ImmunoCAP was 92.3%, and its specificity was 83.3%.
Figures and Tables
Fig. 2
Correlations of specific IgE to Bet v 1 with birch (A), IgE to Mal d 1 with apple (B), and specific IgE to Pru p 1 with peach (C). P-value was applied by Pearson correlation. sIgE, specific IgE; ISA, ISAC standardized units. ISAC (Phadia AB, Uppsla, Sweden), ImmunoCAP (Phadia AB, Uppsala, Sweden).

Fig. 3
Receiver operating characteristic curve for IgE levels by microarray and IgE levels by immunoCAP for diagnosing raw fruit hypersensitivity. AUC, area under curve; SE, standard error; CI, confidence interval. ISAC (Phadia AB, Uppsla, Sweden), ImmunoCAP (Phadia AB, Uppsala, Sweden).
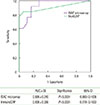
Table 1
Clinical characteristics and specific IgE by ImmunoCAP, skin prick test, allergen components microarray of subjects

References
1. Dreborg S. Food allergy in pollen-sensitive patients. Ann Allergy. 1988; 61(6 Pt 2):41–46.
2. Wuthrich B, Schindler C, Leuenberger P, Ackermann-Liebrich U. Prevalence of atopy and pollinosis in the adult population of Switzerland (SAPALDIA study). Swiss Study on Air Pollution and Lung Diseases in Adults. Int Arch Allergy Immunol. 1995; 106:149–156.

3. Stephen TH, Robert FL, Paul MO, Sujani K, William WB. Pathophysiologic mechanisms of food allergy. In : Adkinson F, Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF, editors. Middleton's allergy: principles and practice. 8th ed. Philadelphia: Mosby;2013. p. 1323.
4. Eriksson NE, Formgren H, Svenonius E. Food hypersensitivity in patients with pollen allergy. Allergy. 1982; 37:437–443.

5. Bircher AJ, Van Melle G, Haller E, Curty B, Frei PC. IgE to food allergens are highly prevalent in patients allergic to pollens, with and without symptoms of food allergy. Clin Exp Allergy. 1994; 24:367–374.

6. Kazemi-Shirazi L, Niederberger V, Linhart B, Lidholm J, Kraft D, Valenta R. Recombinant marker allergens: diagnostic gatekeepers for the treatment of allergy. Int Arch Allergy Immunol. 2002; 127:259–268.

7. Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Gronlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin Exp Allergy. 1999; 29:896–904.

8. Lidholm J, Ballmer-Weber BK, Mari A, Vieths S. Component-resolved diagnostics in food allergy. Curr Opin Allergy Clin Immunol. 2006; 6:234–240.

9. Harwanegg C, Laffer S, Hiller R, Mueller MW, Kraft D, Spitzauer S, et al. Microarrayed recombinant allergens for diagnosis of allergy. Clin Exp Allergy. 2003; 33:7–13.

10. Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, et al. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003; 33:1443–1449.

11. Wöhrl S, Vigl K, Zehetmayer S, Hiller R, Jarisch R, Prinz M, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006; 61:633–639.

12. Ott H, Baron JM, Heise R, Ocklenburg C, Stanzel S, Merk HF, et al. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008; 63:1521–1528.

13. Eigenmann PA. Component-resolved diagnosis in food allergy, are micro-array assays helpful to the clinician. Allergy. 2008; 63:1519–1520.

14. Asero R, Antonicelli L, Arena A, Bommarito L, Caruso B, Colombo G, et al. Causes of food-induced anaphylaxis in Italian adults: a multi-centre study. Int Arch Allergy Immunol. 2009; 150:271–277.

15. Asero R, Pravettoni V. Anaphylaxis to plant-foods and pollen allergens in patients with lipid transfer protein syndrome. Curr Opin Allergy Clin Immunol. 2013; 13:379–385.

16. Deinhofer K, Sevcik H, Balic N, Harwanegg C, Hiller R, Rumpold H, et al. Microarrayed allergens for IgE profiling. Methods. 2004; 32:249–254.

17. Ortolani C, Ispano M, Pastorello E, Bigi A, Ansaloni R. The oral allergy syndrome. Ann Allergy. 1988; 61(6 Pt 2):47–52.
19. Ortolani C, Ballmer-Weber BK, Hansen KS, Ispano M, Wuthrich B, Bindslev-Jensen C, et al. Hazelnut allergy: a double-blind, placebo-controlled food challenge multicenter study. J Allergy Clin Immunol. 2000; 105:577–581.

20. Skamstrup Hansen K, Vestergaard H, Stahl Skov P, Sondergaard Khinchi M, Vieths S, Poulsen LK, et al. Double-blind, placebo-controlled food challenge with apple. Allergy. 2001; 56:109–117.

21. Ballmer-Weber BK, Scheurer S, Fritsche P, Enrique E, Cistero-Bahima A, Haase T, et al. Component-resolved diagnosis with recombinant allergens in patients with cherry allergy. J Allergy Clin Immunol. 2002; 110:167–173.

22. Ballmer-Weber BK, Vieths S, Luttkopf D, Heuschmann P, Wuthrich B. Celery allergy confirmed by double-blind, placebo-controlled food challenge: a clinical study in 32 subjects with a history of adverse reactions to celery root. J Allergy Clin Immunol. 2000; 106:373–378.

23. Ballmer-Weber BK, Wuthrich B, Wangorsch A, Fotisch K, Altmann F, Vieths S. Carrot allergy: double-blinded, placebo-controlled food challenge and identification of allergens. J Allergy Clin Immunol. 2001; 108:301–307.

24. Kleine-Tebbe J, Vogel L, Crowell DN, Haustein UF, Vieths S. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1- related PR-10 protein in soybean, SAM22. J Allergy Clin Immunol. 2002; 110:797–804.

25. García BE, Lizaso MT. Cross-reactivity syndromes in food allergy. J Investig Allergol Clin Immunol. 2011; 21:162–170.
26. González-Mancebo E, Gonzalez-de-Olano D, Trujillo MJ, Santos S, Gandolfo-Cano M, Melendez A, et al. Prevalence of sensitization to lipid transfer proteins and profilins in a population of 430 patients in the south of Madrid. J Investig Allergol Clin Immunol. 2011; 21:278–282.
27. Pascal M, Munoz-Cano R, Reina Z, Palacin A, Vilella R, Picado C, et al. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy. 2012; 42:1529–1539.

28. Pastorello EA, Farioli L, Pravettoni V, Scibilia J, Mascheri A, Borgonovo L, et al. Pru p 3-sensitised Italian peach-allergic patients are less likely to develop severe symptoms when also presenting IgE antibodies to Pru p 1 and Pru p 4. Int Arch Allergy Immunol. 2011; 156:362–372.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download