Abstract
Trimethoprim-sulfamethoxazole (TMP-SMX) is an antibiotic used for the treatment or prophylaxis of Pneumocystis pneumonia and other infectious conditions. Sulfonamide derivatives have been reported to cause delayed hypersensitivity reactions, resulting in switch to less effective second-line antibiotics. Although desensitization is traditionally known to be effective in patients with immediate hypersensitivity, it is also applied to the treatment of delayed hypersensitivity in recent years. A 66-year-old female who had a history of repeated TMP-SMX-induced delayed hypersensitivity presenting as whole body rashes needed to take prophylactic dose of TMP-SMX (80/400 mg daily) before initiation of chemotherapy for multiple myeloma. Intravenous rapid desensitization was performed by using a 11-step, 4-bottle protocol from 1:1,000 to 1:1 solution for 3 hours to reach the target dose for prophylaxis. After successful rapid desensitization of TMP-SMX, 1-month prophylaxis was completed without any complications until the patient recovered normal immunity. We herein reported a case of delayed hypersensitivity reaction to TMP-SMX in an about-to-be immunocompromised host with planned chemotherapy who successfully completed 1-month prophylaxis with the drug without any complications through rapid desensitization.
Figures and Tables
Table 1
The preparation of 4-bottle tenfold increasing solutions (A, B, C, and D) used in the rapid desensitization protocol
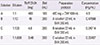
Table 2
The 11-step desensitization protocol used in the present case of the TMP/SMX induced delayed hypersensitivity

References
1. Bang J, Shin H. Guidance of antibiotic therapy. 3rd ed. Seoul: MIP;2008.
2. Pichler WJ. Drug hypersensitivity. Basel: Karger;2007.
3. Douglas R, Spelman D, Czarny D, O'Hehir RE. Successful desensitization of two patients who previously developed Stevens-Johnson syndrome while receiving trimethoprim-sulfamethoxazole. Clin Infect Dis. 1997; 25:1480.

4. Pyle RC, Butterfield JH, Volcheck GW, Podjasek JC, Rank MA, Li JT, et al. Successful outpatient graded administration of trimethoprim-sulfamethoxazole in patients without HIV and with a history of sulfonamide adverse drug reaction. J Allergy Clin Immunol Pract. 2014; 2:52–58.

5. Patriarca G, Schiavino D, Buonomo A, Aruanno A, Altomonte G, Nucera E. Desensitization to co-trimoxazole in a patient with fixed drug eruption. J Investig Allergol Clin Immunol. 2008; 18:309–311.
6. Kim MS, Cho YJ. Cyclosporine desensitization in patient with multiple hypersensitivity reactions immediately after peripheral blood stem cell transplantation. Korean J Asthma Allergy Clin Immunol. 2008; 28:59–63.
7. Yoshizawa S, Yasuoka A, Kikuchi Y, Honda M, Gatanaga H, Tachikawa N, et al. A 5-day course of oral desensitization to trimethoprim/sulfamethoxazole (T/S) in patients with human immunodeficiency virus type-1 infection who were previously intolerant to T/S. Ann Allergy Asthma Immunol. 2000; 85:241–244.

8. Lee AR, Kim SJ, Kim J, Park JH, Lee JK, Kim JY, et al. Successful desensitization for antitubercular drugs. Allergy Asthma Respir Dis. 2013; 1:395–399.

9. Scherer K, Brockow K, Aberer W, Gooi JH, Demoly P, Romano A, et al. Desensitization in delayed drug hypersensitivity reactions: an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013; 68:844–852.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download