Abstract
Objective
The purpose of this study was to investigate the impact of continuous renal replacement therapy (CRRT) on survival and relevant factors in patients who underwent CRRT after traumatic brain injury (TBI).
Methods
We retrospectively reviewed the laboratory, clinical, and radiological data of 29 patients who underwent CRRT among 1,190 TBI patients treated at our institution between April 2011 and June 2015. There were 20 men and 9 women, and the mean age was 60.2 years. The mean initial Glasgow Coma Scale score was 9.2, and the mean injury severity score was 24. Kaplan-Meier method and Cox regression were used for analysis of survival and relevant factors.
Results
The actuarial median survival time of the 29 patients was 163 days (range, 3-317). Among the above 29 patients, 22 died with a median survival time of 8 days (range, 3-55). The causes of death were TBI-related in 8, sepsis due to pneumonia or acute respiratory distress syndrome (ARDS) in 4, and multi-organ failure in 10. Among the various factors, urine quantity of more than 500 mL for 24-hours before receiving CRRT was a significant and favorable factor for survival in the multivariate analysis (p=0.026).
Conclusion
According to our results, we suggest that early intervention with CRRT may be beneficial in the treatment of TBI patients with impending acute renal failure (ARF). To define the therapeutic advantages of early CRRT in the TBI patients with ARF, a well-designed and controlled study with more cases is required.
Despite the implementation of clinical guidelines and improvements in the critical care of patients with traumatic brain injury (TBI), the prognosis of patients with severe TBI has remained poor during the last decade.16) The mortality rate of severe TBI is known to be about 32% to 52%, and about 6% to 11% of patients become vegetative or are severely disabled.313)
It has been reported that dysfunction of at least one non-neurologic organ system develop in about 89% of patients with severe TBI. Among them, renal dysfunction occurred in 7% of patients. The incidence of renal failure in TBI has been reported as 0.45% to 1.9%.1217) Acute renal failure (ARF) may occur by ischemic renal injury, sepsis or administration of nephrotoxic agents in TBI patients, and can be fatal to these patients. Therefore, renal replacement therapy is required for TBI patients with ARF to decrease post-traumatic mortality. Among treatment modalities for ARF, continuous renal replacement therapy (CRRT) has shown to have a much lesser effect on intracranial pressure (ICP) than intermittent hemodialysis (IHD).6) Additionally, some authors have reported that CRRT have an effect on lowering ICP.89) Regarding hemodynamics, CRRT can be a better treatment modality for ARF compared to IHD in severe TBI patients.
However, little has been reported about the treatment results of CRRT on TBI patients with ARF. We aimed to investigate the outcomes of CRRT on TBI patients in whom ARF developed after trauma, and to study relevant factors which influence patients’ survival.
Between April 2011 and June 2015, 1,190 TBI patients were treated at our institution. Among them, 29 patients underwent renal replacement therapy for ARF. We retrospectively reviewed the laboratory, clinical, and radiological data of those 29 patients.
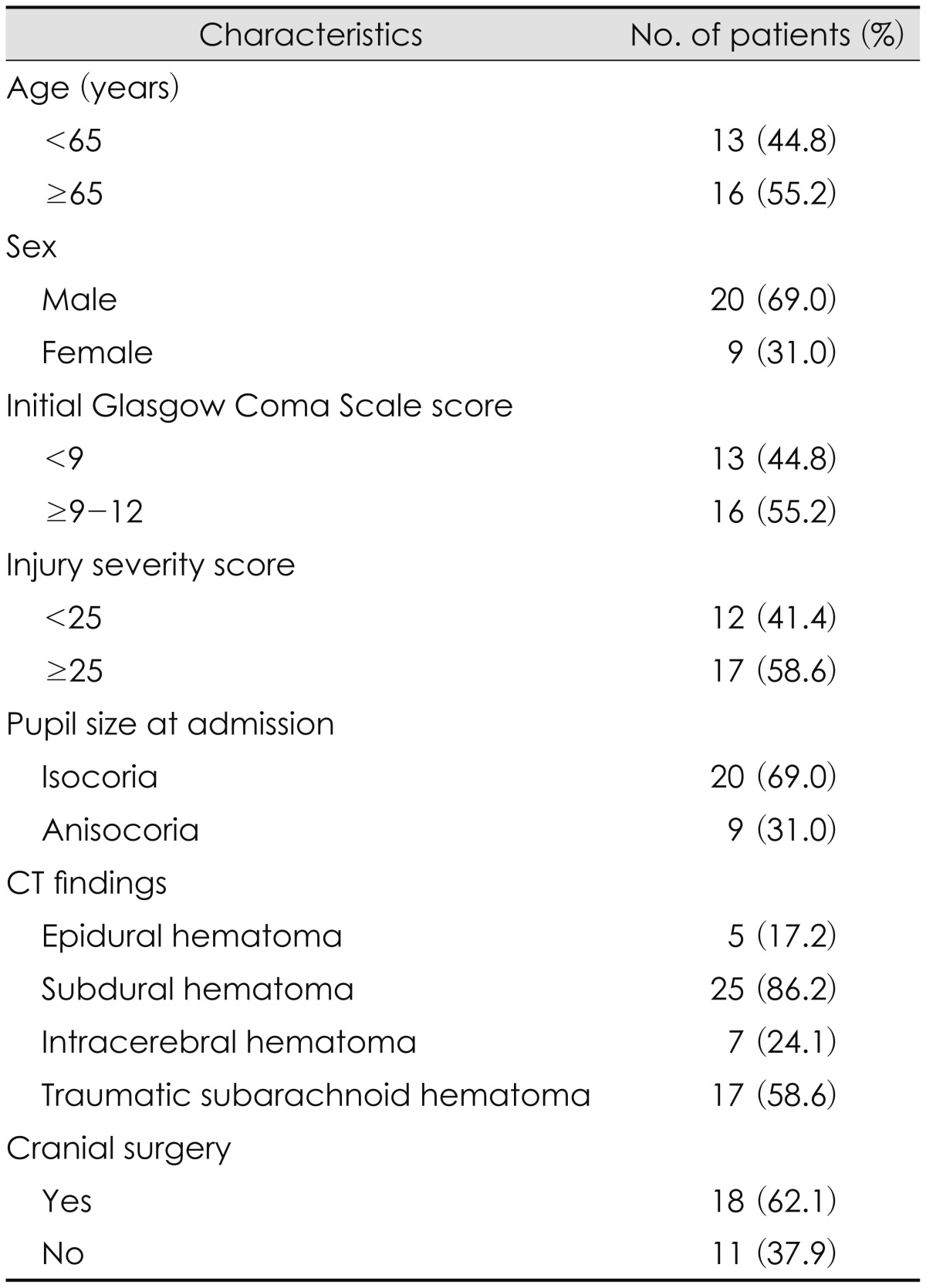
Initial Glasgow Coma Scale (GCS) score was checked when the patient was arrived to the emergency room. Injury severity score (ISS) has six categories to head and neck, face, chest, abdomen or pelvic contents, extremities or pelvic girdle, and external. ISS scores ranges from 1 to 75 (ISS scores of 5 for each category). There were 20 men and 9 women. The mean age at admission was 60.2 years (range, 16-84). The mean initial GCS score was 9.2 (range, 3-15), and the mean ISS was 24 (range, 9-59). Pupil size at admission was isocoric in 20 patients, and anisocoric in 9. Brain computed tomography (CT) scan showed epidural hematoma (EDH), subdural hematoma (SDH), intracerebral hemorrhage (ICH), and traumatic subarachnoid hemorrhage (t-SAH) in 5, 25, 7, and 17 patients, respectively. Eighteen out of 29 patients underwent surgical intervention for treatment of TBI (Table 1).
All 29 patients had normal kidney function on admission. However, 3 patients had a past history of chronic kidney disease. When the patients’ urine output decreased, we checked the serum creatinine level and calculated the glomerular filtration rate. CRRT was started when the serum creatinine level increased to more than twice of the basal level, and the urine output decreased to less than 30 mL per hour despite hydration and diuretics. The mean serum creatinine level was 1.03 mg/dL (range, 0.6-5.0) at admission and 2.79 mg/dL (range, 1.6-7.9) before starting CRRT. CRRT was maintained until serum creatinine level was normalized (0.7-1.2 mg/dL).
The median starting date of CRRT was 3 days (range, 0-34) after TBI. The median CRRT period was 5 days (range, 0-288). If the patients' vital sign was stable, more than 100 mL/hour (>2400 mL/day) of dialysis volume was removed.
We investigated survival time of 29 TBI patients who underwent CRRT and analyzed the relationship between survival time and the laboratory, clinical, and radiological factors. Survival factors were categorized as follows; age (≥65 vs. <65), 24-hours urine output (≥500 mL vs. <500 mL) before receiving CRRT, ISS (<25 vs. ≥25), the presence of SDH, initial GCS score (<9 vs. ≥9), pupil size at admission (isocoria vs. anisocoria), cranial surgery (yes vs. no), and intracranial hemorrhage (EDH, SDH, ICH, t-SAH absent vs. present).
Statistical analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). To investigate relevant factors, Kaplan-Meier analysis was used and survival outcome was compared using the log-rank sum test for categorical variables. Cox proportional hazards model was used for multivariate analysis. Results were regarded as significant at p<0.05.
The actuarial median survival time of the 29 patients was 163 days after trauma (range, 3-317). Among the 29, 22 patients died with a median survival time of 8 days (range, 3-55) (Figure 1A).
The causes of death were TBI-related in 8, sepsis due to pneumonia or acute respiratory distress syndrome (ARDS) in 4, and multi-organ failure in 10.
Among the various factors related to survival, 24-hr urine output before receiving CRRT (≥500 mL vs. <500 mL) (Figure 1B), the presence of SDH (Figure 1C), and ISS (<25 vs. ≥25) were significant after univariate analysis (Figure 1D, Table 2).
Median survival time was 10 days (range, 3-136) when the patients’ urine output was less than 500 mL per day before receiving CRRT, whereas more than half of the patients survived when the urine output was 500 mL per day or more (p=0.015). Of the nine patients who had a 24-hours urine output of more than 500 mL per day before receiving CRRT, 4 (44.4%) patients died (TBI-related brain herniation in 1, multi-organ failure in 3), with a median survival time of 146 days (range, 4-317). Of the 20 patients who had a 24-hours urine output of less than 500 mL per day before receiving CRRT, 18 (90.0%) patients died (TBI-related brain herniation in 7, multi-organ failure in 11), with a median survival time of 10 days (range, 3-136 days) (Figure 1B).
For the patient with SDH, the median survival time was 10±2 days, whereas more than half of the patients survived when SDH was absent (p=0.030) (Figure 1C). Median survival time was 25±29 days when the ISS score was less than 25, whereas it was 7±3 days when the ISS score was 25 or more (p=0.047) (Figure 1D).
In multivariate analysis, only 24-hours urine output before receiving CRRT (≥500 mL vs. <500 mL) remained as a significant factor (p=0.026) (Table 2).
TBI patients are often exposed to multiple trauma, sepsis, and many nephrotoxic drugs. Therefore, ARF can occur after hypotensive acute tubular necrosis (ATN), vasomotor ATN, and toxic ATN after TBI.5) Urea and other solutes are increased in ARF patients and these solutes can pass into the brain because the blood-brain barrier (BBB) breaks down in patients with TBI. This influx is initially compensated by astrocytes taking up additional ions and water. However, this compensation mechanism is disrupted in TBI, thereby cerebral edema can become more worse.1211) Additionally, ARF can alter the concentration of neurotransmitters or circulating cytokines, acid-base balance, hemostasis, and drug metabolism. Increased circulating cytokines can lead to disruption of the BBB, allowing increased access to cytotoxic inflammatory cells, cytokines, complement, amino acids, and organic osmolytes.14) Therefore, it has been reported that TBI patients with ARF have a higher incidence of poor outcome when compared with TBI patients without renal dysfunction.12)
Renal replacement therapy for ARF in the patient with TBI is challenging, because conventional IHD is known to lead to an increase in brain water content, even in non-TBI patients undergoing regular hemodialysis.5) Compared to standard IHD, CRRT has been shown to result in greater intracranial stability.615) During treatment with CRRT, changes in osmolality and changes of urea and bicarbonate levels were much less than those during IHD.5) This intracranial stability can be achieved by improved cardiovascular stability, because various modes of CRRT can be selected according to cardiovascular volume status.4) Therefore, it can be stated that CRRT has many advantages over conventional IHD in the treatment of ARF for patients with TBI.
The Acute Kidney Injury Network working group summarized the available evidence and presented absolute indications for initiation of CRRT as follows; a serum urea concentration >224 mg/dL (blood urea nitrogen [BUN] >100 mg/dL), hyperkalemia (>6 mEq/L and electrocardiogram abnormalities), hypermagnesemia (>8 mEq/L), severe acidosis (pH <7.15), lactic acidosis related to metformin use and anuria with diuretic resistant volume overload.710) However, to our knowledge, there are no previous reports regarding indications for initiation of CRRT for ARF in patients with TBI. In our study, it was found that starting early CRRT before when the urine output is reduced to less than 500 mL per day significantly prolonged survival time of TBI patients with post-traumatic ARF. Our result suggests that starting CRRT in the early stage of ARF is required to improve the survival rate of TBI patients who have worse outcomes compared to those without post-traumatic ARF.
This study has some limitations of a retrospective design, a small number of patients, and a non-comparative study. However, to the best of our knowledge, this is the first analysis of outcomes of CRRT in TBI patients with post-traumatic ARF. Further studies with a prospective design, many more cases, and control group with conventional IHD are necessary to prove the beneficial therapeutic effects of CRRT compared to conventional IHD, and the best timing of initiation of CRRT in the TBI patients with post-traumatic ARF.
According to our results, we suggest that early intervention with CRRT before urine output is reduced to less than 500 mL per day may be beneficial in the treatment of TBI patients with impending ARF. To define the therapeutic advantages of early CRRT in the TBI patients with ARF, a well-designed and controlled study with more cases is required.
References
1. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006; 7:41–53. PMID: 16371949.

2. Beaumont A, Marmarou A, Hayasaki K, Barzo P, Fatouros P, Corwin F, et al. The permissive nature of blood brain barrier (BBB) opening in edema formation following traumatic brain injury. Acta Neurochir Suppl. 2000; 76:125–129. PMID: 11449990.

3. Becker DP, Miller JD, Ward JD, Greenberg RP, Young HF, Sakalas R. The outcome from severe head injury with early diagnosis and intensive management. J Neurosurg. 1977; 47:491–502. PMID: 903803.

4. Davenport A. Is there a role for continuous renal replacement therapies in patients with liver and renal failure? Kidney Int Suppl. 1999; S62–S66.

5. Davenport A. Renal replacement therapy in the patient with acute brain injury. Am J Kidney Dis. 2001; 37:457–466. PMID: 11228168.

6. Davenport A, Will EJ, Losowsky MS, Swindells S. Continuous arteriovenous haemofiltration in patients with hepatic encephalopathy and renal failure. Br Med J (Clin Res Ed). 1987; 295:1028.

7. De Corte W, Vanholder R, Dhondt AW, De Waele JJ, Decruyenaere J, Danneels C, et al. Serum urea concentration is probably not related to outcome in ICU patients with AKI and renal replacement therapy. Nephrol Dial Transplant. 2011; 26:3211–3218. PMID: 21421593.

8. Fletcher JJ, Bergman K, Carlson G, Feucht EC, Blostein PA. Continuous renal replacement therapy for refractory intracranial hypertension? J Trauma. 2010; 68:1506–1509. PMID: 20539193.

9. Fletcher JJ, Bergman K, Feucht EC, Blostein P. Continuous renal replacement therapy for refractory intracranial hypertension. Neurocrit Care. 2009; 11:101–105. PMID: 19267223.

10. Gibney N, Hoste E, Burdmann EA, Bunchman T, Kher V, Viswanathan R, et al. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clin J Am Soc Nephrol. 2008; 3:876–880. PMID: 18322044.

11. Härtl R, Medary M, Ruge M, Arfors KE, Ghajar J. Blood-brain barrier breakdown occurs early after traumatic brain injury and is not related to white blood cell adherence. Acta Neurochir Suppl. 1997; 70:240–242. PMID: 9416334.

12. Li N, Zhao WG, Zhang WF. Acute kidney injury in patients with severe traumatic brain injury: implementation of the acute kidney injury network stage system. Neurocrit Care. 2011; 14:377–381. PMID: 21298359.

13. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008; 7:728–741. PMID: 18635021.

14. Nongnuch A, Panorchan K, Davenport A. Brain-kidney crosstalk. Crit Care. 2014; 18:225. PMID: 25043644.

15. Ronco C, Bellomo R, Brendolan A, Pinna V, La Greca G. Brain density changes during renal replacement in critically ill patients with acute renal failure. Continuous hemofiltration versus intermittent hemodialysis. J Nephrol. 1999; 12:173–178. PMID: 10440514.
16. Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013; 9:231–236. PMID: 23443846.

17. Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Nonneurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005; 33:654–660. PMID: 15753760.

FIGURE 1
Kaplan-Meier curves for the patients underwent continuous renal replacement therapy (CRRT) after traumatic brain injury. (A) Overall survival. (B) Twenty four hours urine output before receiving CRRT. (C) Subdural hematoma. (D) Injury severity score. SDH: subdural hematoma.
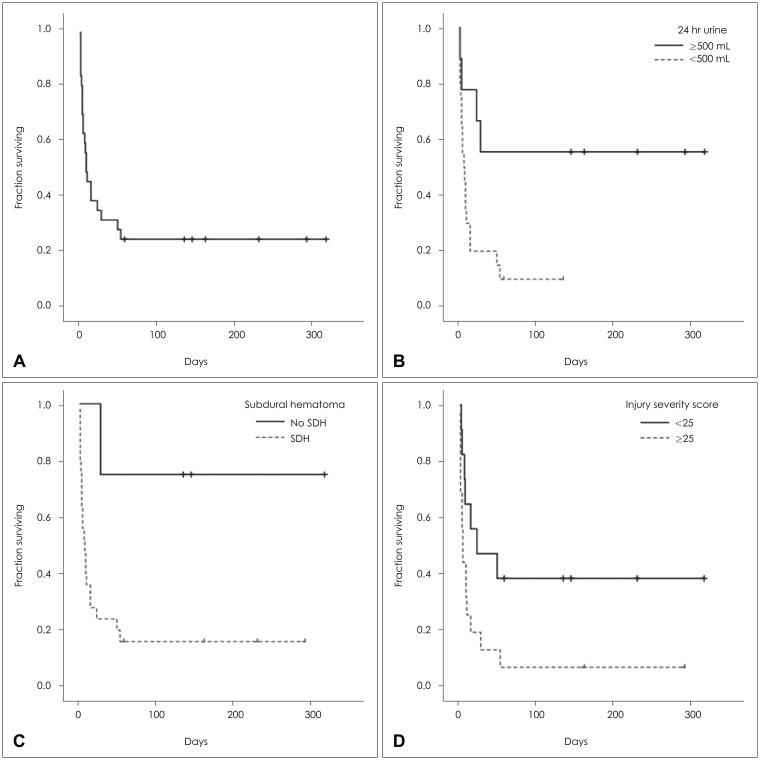
TABLE 2
Factors related to survival time
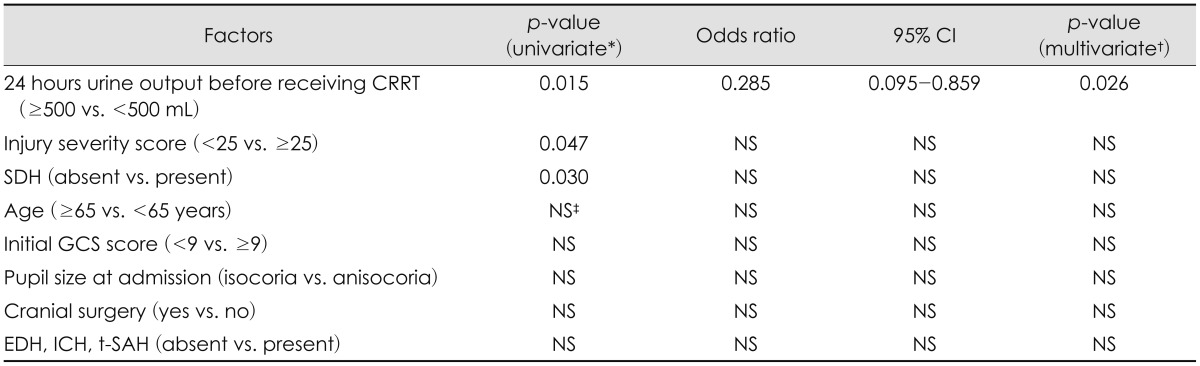
*Kaplan-Meier analysis with log-rank sum test, †Cox proportional hazards model, ‡non-significant. Significant at p<0.05. CI: confidence interval, CRRT: continuous renal replacement therapy, NS: non-significant, SDH: subdural hematoma, GCS: Glasgow Coma Scale, EDH: epidural hematoma, ICH: intracerebral hemorrhage, t-SAH: traumatic subarachnoid hemorrhage




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download