Abstract
Objective
Therapeutic hypothermia (TH) and decompressive craniectomy are neuroprotective interventions following severe brain swelling. The precise benefits, risks, and clinical outcomes in brain swelling after TH are still being investigated. We aimed to investigate the effects of TH in severe brain injury after decompressive craniectomy.
Methods
We reviewed the cases of 24 patients who underwent decompressive craniectomy with intracranial pressure (ICP) monitor insertion in one medical center between January 2012 and May 2016. All patients had an ICP greater than 15 mmHg and a Glasgow Coma Scale score of less than 7 at the time of intervention. TH was induced in half of the patients (n=12) directly after surgery; the remaining 12 patients remained normothermic. The ICP, vital signs, complications, and functional outcomes were reviewed and compared between the patient groups.
Results
The mean ICP in the TH group was significantly lower than in the normothermia group. Complications during the 3 days after surgery were not different between the groups, with the exception of hypokalemia in the TH group. Mortality in the intensive care unit (ICU) was higher in the normothermia group, but the functional outcomes 3 months after surgery were not different between the TH and normothermia groups.
Intracranial pressure (ICP) monitoring has a critical role in predicting mortality of patients with severe brain swelling.5) Active treatment for elevated ICP reduces mortality and improves outcomes.1216) Therapeutic hypothermia (TH) is one option for treating severe brain swelling;9) this method cools the core temperature of a patient to 32 to 35℃, which protects the brain from secondary injury. TH is believed to improve neurological outcomes by decreasing production of free radicals, protecting the blood-brain barrier, attenuating ionic disruption, and inhibiting the release of excitatory amino acids.13) However, even in recent studies, the effectiveness of TH is controversial.36) Several clinical trials of TH as a neuroprotective intervention in severe traumatic brain injury have been conducted,231015) but the use of TH for patients with elevated ICP after surgical decompression has not been extensively evaluated.114) The goal of this study is to assess outcomes for patients who had undergone TH after surgical decompression for severe brain swelling and to investigate the relationship between TH and ICP, in terms of effectiveness and complications.
We reviewed patients who were treated for severe brain swelling at a single care center between January 2012 and May 2016. Since we have been started TH from March 2014, patients of TH group was selected from whom visited our center between March 2014 and May 2016. Control group was selected from whom visited our center between January 2012 and March 2014 with corresponding characteristics to TH group. All patients underwent decompressive craniectomy due to severe brain swelling or large hematoma, which can elevate ICP. The frontotemporoparietal bone flap was removed during surgery. A 'Spiegelberg parenchymal Probe 3PN' device was inserted during surgery and 'ICP-Monitor HDM 26.1' was used to monitor ICP. After surgery, computed tomography (CT) was conducted and ICP was measured. For this study, we selected patients whose age range from 18 to 80, and had poor consciousness [Glasgow Coma Scale (GCS) 3<score≤7] with elevated ICP (>15 mmHg). We excluded patients with predisposing infection, coagulopathy, and pregnancy. Patients presenting hemodynamic instability, comatose state, and severe ICP elevation (>40 mmHg) at postoperative period were also excluded. And patients whose guardians did not agree for aggressive treatment nor TH had been excluded from selection. None of the patients had another surgery during this admission. Patients received conservative therapy for controlling ICP, including regular injections of mannitol (0.25 mg/kg bolus), elevating the head to 30° from the horizontal position, and ventilating to normocarbia (PaCO2=35-40 mmHg). Mild sedation with remifentanyl (l mcg/kg/min) and propofol (75 mcg/kg/min) was implemented if patients did not tolerate the ventilator. Mean blood pressure was maintained between 90 and 100 mmHg and hypotension was avoided. Volume maintenance was controlled by maintaining the central venous pressure between 8 and 12 mmHg. ICP, blood pressure, and core temperature were monitored every hour. Laboratory values, including complete blood cell count, electrolytes, amylase, lipase, and blood coagulation tests, were monitored every 6 hours. The following abnormal laboratory findings were recorded: hypernatremia (serum sodium >150 mmol/L), hypokalemia (serum potassium <3.5 mmol/L), abnormal coagulation factor (international normalized ratio >1.2 or activated partial thromboplastin time >45 seconds), and pancreatitis (amylase >100 U/L or lipase >60 U/L). A chest X-ray and electrocardiogram were obtained daily to detect signs of pneumonia or arrhythmia. All patients received a modified Rankin Scale (mRS) score 3 months after the brain injury.
If TH was determined to be appropriate, induction of TH was completed by a full drip of frozen normal saline via intravenous peripheral lines and a Foley catheter. External cooling devices (ArcticSun® 5000 Temperature Management System and ArcticGel™ Pads; Medivance, Louisville, CO, USA) were used to induce and maintain cooling until the patient's core temperature reached 33 to 34℃. The core temperature was measured by esophageal probe and closely monitored every hour. Remifentanyl and propofol by intravenous continuous injection, as mentioned above, were used for sedation during TH. Vecuronium (1.2 mcg/kg/min) was considered when patients showed shivering.4) TH was maintained for 72 hours. After 72 hours of TH, the patient's body was rewarmed at 0.5℃ per hour until it reached 36.5℃. And CT was followed after rewarming is done.
Statistical analyses were completed using the SPSS software, version 23 (SPSS Inc., Chicago, IL, USA). We used the chi-square test or Fischer's exact test to compare TH patients and normothermic controls. We used 2-sided t-tests to compare simple continuous variables. All p-values less than 0.05 were considered to be statistically significant.
We reviewed a total of 24 patient records. All of the patients had undergone decompressive craniectomy due to severe brain swelling. TH was induced in half (n=12) of the patients immediately after surgery. And correspondent 12 patients who were maintained normal core temperature were selected (Table 1).
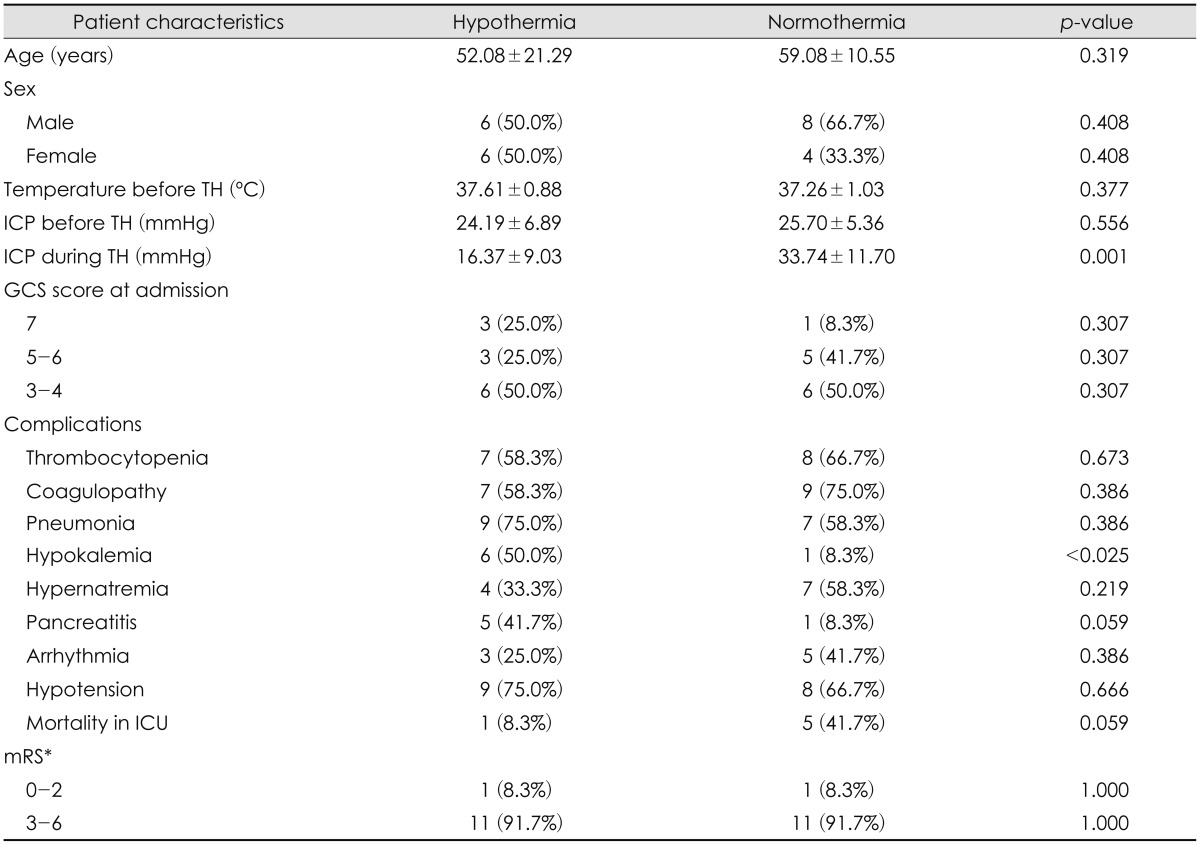
Clinical findings and outcomes between the TH and normothermia groups are compared (Table 2). The mean age of the TH group was 52.08±21.29 years (range, 19-75 years), and the mean age of the normothermia group was 59.08±10.55 years (range, 45-80 years). The average core temperature of the TH group was 37.61±0.88℃ before induction of TH, and the temperature was maintained at 33 to 34℃ during TH. The average core temperature of the normothermia group was 37.26±1.03℃.
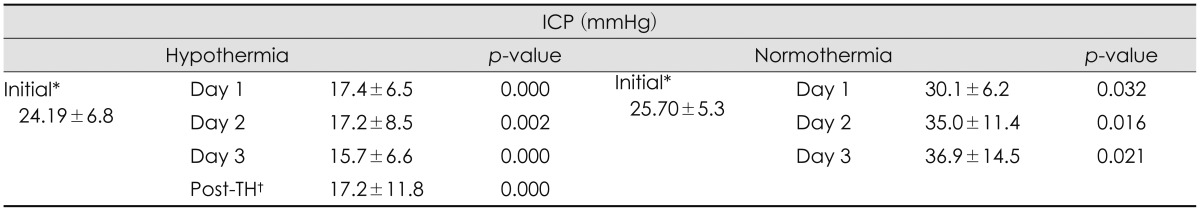
Both groups showed elevated ICP (>15 mmHg) after surgery. The ICP obtained immediately after surgery of the TH group was 24.19±6.89 mmHg, and the ICP of the control group was 25.70±5.36 mmHg. During TH, the mean ICP of the TH group was 16.37±9.03 mmHg, which represents a decrease in ICP compared with ICP before TH. The mean ICP of the normothermia group during the 3 days after the surgery was 33.74±11.70 mmHg, which represents an increase in ICP directly after surgery (Table 2). We measured daily ICP changes in the TH and normothermia groups (Table 3). We observed small but significant decreases in ICP each day in TH group, and daily ICP changes in the normothermia group showed increases each day. The mean GCS score before surgery of TH group was 5 and normothermia group was 4.88. The mean GCS score was slightly lower in the normothermia group but there was no statistical significance (p=0.758).
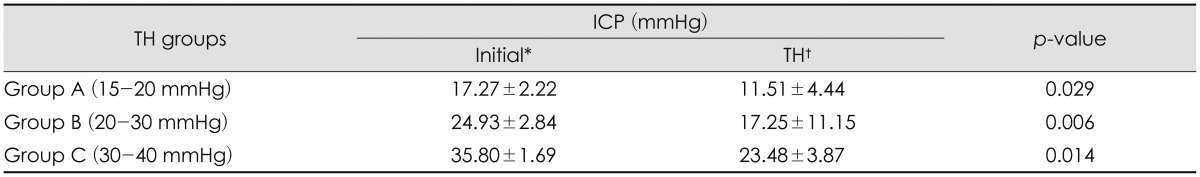
We also compared mean ICP changes and correlated the changes with time of TH (Table 4). We divided the TH group into 3 groups according to ICP after decompressive craniectomy: 15 to 20 mmHg (Group A), 20 to 30 mmHg (Group B), and 30 to 40 mmHg (Group C). Each group showed significant decrease in ICP. When comparing each groups, Group C showed a significantly larger decrease in ICP than Group A and B.
There was no significant difference in the occurrence of complications between the TH and normothermia groups. However, hypokalemia was noted more often in the TH group than in the normothermia group (n=6/12 vs. n=1/12, respectively). The mortality rate of patients in the intensive care unit (ICU) was different between groups: only 1 patient in the TH group died, and 5 died in the normothermia group. Only 1 patient in the TH group and 1 patient in the normothermia group had good mRS scores (lower than 2) 3 months after brain injury (Table 1).
Many clinical trials and review articles of TH have been published. The Eurotherm 3235 Trial8) was a large, randomized trial, and its results suggest that TH is beneficial for lowering ICP in patients with traumatic brain injury and ICP higher than 20 mmHg. This clinical trial is still ongoing. However, other recent studies suggest that TH has no benefit for clinical outcomes. Andrews et al.3) reported that TH plus standard care for traumatic brain injury successfully reduced ICP, but functional recovery did not improve compared with standard care alone. Dietrich and Bramlett6) reported that TH has been successfully tested in several single-institution, clinical traumatic brain injury studies, but larger, randomized, multicenter trials have failed to show benefits of TH. Therefore, TH is still controversial and more research is needed to establish proper protocols for its use.
Limited research has evaluated the benefits of combination therapy with TH and decompressive craniectomy for severe brain injury. Allahtavakoli et al.1) studied the benefits of TH and decompressive craniectomy after acute stoke in rats, and they reported that the combination of TH and decompressive craniectomy was more efficient than either technique alone. Els et al.7) also suggested that the combination of TH and decompressive craniectomy was effective for malignant ischemic stroke and improved functional outcomes.
In our study, initial ICP after decompressive craniectomy was similar in both the TH group and the normothermia group. After TH was induced, there were significant differences in ICP between the groups (p=0.000). The TH group showed a decrease in ICP, and these lower values were maintained during TH; the normothermia group showed a significant, severe increase in ICP, which can lead to secondary brain injury. It may be predicted to result from production of free radicals, destruction of the blood-brain barrier, aggravating ionic disruption, and releasing of excitatory amino acids those induced from brain injury.13) And as mentioned above, TH is believed to improve those neurological outcomes. The daily mean ICP values during TH were similarly decreased, and these changes were statistically significant (p=0.002). Patients in the TH group with the high ICP (20-40 mmHg) before TH showed significant decreases during TH. We observed no rebound elevation of ICP after normalization of core temperature (Table 3). These results suggest that TH is efficient for reducing ICP and can be helpful in cases of malignant brain swelling.
Hypokalemia was the only complication that occurred more frequently in the TH group than in the normothermia group. Other well-known complications of TH, such as thrombocytopenia, coagulopathy, pneumonia, electrolyte imbalance, and cardiac problems, occurred with similar incidences in both groups. This may be due to the fact that all patients had severe brain injury that required surgery, and these complications can result from the surgery itself or from secondary issues induced by severe brain injury.11) Overall, we conclude that TH is effective in managing the early phase of severe brain swelling and controlling ICP.
More patients in the normothermia group died in the ICU than in the TH group, but the difference was not statistically significant. These patients may have died due to the severe brain injury. Also, the lack of significance may be due to the small sample size, which included only 12 patients in each group. Still, we cannot rule out the efficacy of TH for improving mortality, and we recommend further investigation of this outcome. Overall, in our study, TH showed positive results in the early management of severe brain injury, but both the TH group and the normothermia group showed poor functional outcomes. Each group had only 1 patient who had a mRS score of less than 2 (i.e., a score indicating that patients were able to carry out all activities with slight disability). In current clinical trials, the decision to induce TH is based on early ICP monitoring and it is used for neuroprotective purposes.3) And surgical treatments are typically the second choice for controlling ICP. But, in our study, all patients had severe brain injury that required surgery. Therefore, the severity of brain injury in our study could have been worse than in other studies, which may have led to poorer prognoses and outcomes. Additional research of post-operative TH management should be conducted.
In our study, patients were treated according to the surgeons' judgment, and causes of brain swelling were not same. We conducted this study with a retrospective design, and it was not a randomized trial. These factors may have contributed to selection bias.
Very few studies have been conducted about TH for post-operative care in severe brain injury. We suggest that TH is efficient and beneficial in the early management of post-operative care in patients with severe brain swelling and that TH improves mortality. TH is more effective in patients with higher ICP than in patients with lower ICP.
References
1. Allahtavakoli M, Kahnouei MH, Rezazadeh H, Roohbakhsh A, Mahmoodi MH, Moghadam-Ahmadi A, et al. Delayed combination therapy of local brain hypothermia and decompressive craniectomy on acute stroke outcome in rat. Iran J Basic Med Sci. 2014; 17:476–482. PMID: 25429337.
2. Andrews PJ, Sinclair HL, Battison CG, Polderman KH, Citerio G, Mascia L, et al. European society of intensive care medicine study of therapeutic hypothermia (32-35 degrees C) for intracranial pressure reduction after traumatic brain injury (the Eurotherm3235Trial). Trials. 2011; 12:8. PMID: 21226939.

3. Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, et al. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N Engl J Med. 2015; 373:2403–2412. PMID: 26444221.

4. Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008; 39:3242–3247. PMID: 18927450.
5. Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012; 367:2471–2481. PMID: 23234472.

6. Dietrich WD, Bramlett HM. Therapeutic hypothermia and targeted temperature management in traumatic brain injury: Clinical challenges for successful translation. Brain Res. 2016; 1640:94–103. PMID: 26746342.

7. Els T, Oehm E, Voigt S, Klisch J, Hetzel A, Kassubek J. Safety and therapeutical benefit of hemicraniectomy combined with mild hypothermia in comparison with hemicraniectomy alone in patients with malignant ischemic stroke. Cerebrovasc Dis. 2006; 21:79–85. PMID: 16330868.

8. Flynn LM, Rhodes J, Andrews PJ. Therapeutic hypothermia reduces intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury: preliminary data from the Eurotherm 3235 Trial. Ther Hypothermia Temp Manag. 2015; 5:143–151. PMID: 26060880.
9. Harris OA, Colford JM Jr, Good MC, Matz PG. The role of hypothermia in the management of severe brain injury: a meta-analysis. Arch Neurol. 2002; 59:1077–1083. PMID: 12117354.
10. Kollmar R, Blank T, Han JL, Georgiadis D, Schwab S. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke. 2007; 38:1585–1589. PMID: 17363720.
11. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006; 21:375–378. PMID: 16983222.
12. Park JH, Park JE, Kim SH, Lim YC, You NK, Ahn YH, et al. Outcomes of ultra-early decompressive craniectomy after severe traumatic brain injury-Treatment outcomes after severe TBI. Korean J Neurotrauma. 2014; 10:112–118. PMID: 27169044.

13. Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med. 1998; 26:562–567. PMID: 9504587.

14. Timofeev I, Czosnyka M, Nortje J, Smielewski P, Kirkpatrick P, Gupta A, et al. Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J Neurosurg. 2008; 108:66–73. PMID: 18173312.

15. Tokutomi T, Morimoto K, Miyagi T, Yamaguchi S, Ishikawa K, Shigemori M. Optimal temperature for the management of severe traumatic brain injury: effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics, and metabolism. Neurosurgery. 2003; 52:102–111. PMID: 12493106.

16. Vik A, Nag T, Fredriksli OA, Skandsen T, Moen KG, Schirmer-Mikalsen K, et al. Relationship of "dose" of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg. 2008; 109:678–684. PMID: 18826355.

TABLE 1
Clinical characteristics and outcomes of patients treated with therapeutic hypothermia and normothermia groups
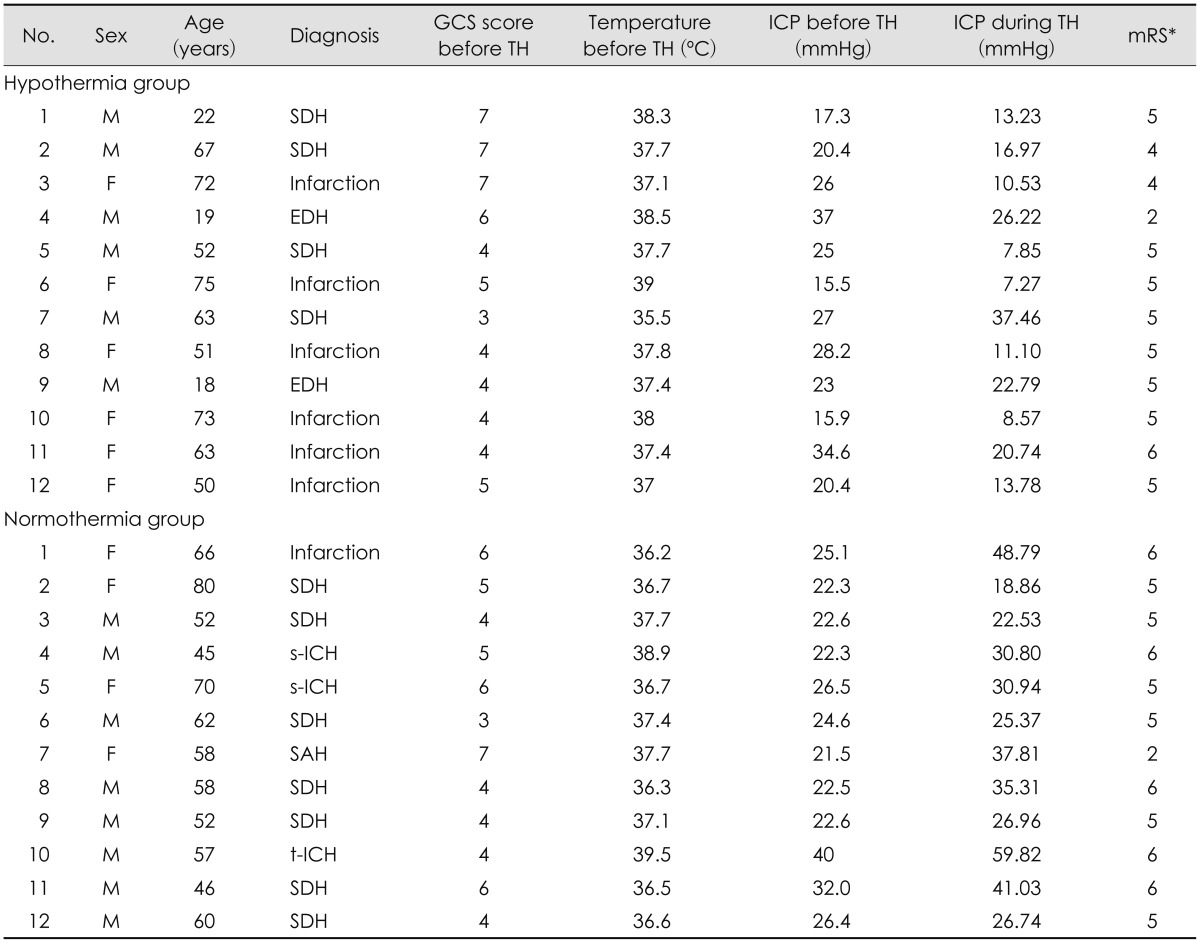
implement of therapeutic hypothermia, *mRS was evaluated 3 months after surgery. GCS: Glasgow Coma Scale, TH: therapeutic hypothermia, ICP: intracranial pressure, mRS: modified Rankin Score, M: male, F: female, SDH: subdural hematoma, EDH: epidural hematoma, s-ICH: spontaneous intracerebral hemorrhage, SAH: subarachnoid hemorrhage, t-ICH: traumatic intracerebral hemorrhage




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download