Abstract
Objective
Chronic subdural hematoma (CSDH) is a condition mostly present in older people. Men are more commonly affected than women. Several theories about male predominance could not enough to explain the reason for male predominance on CSDH. The purpose of this study is to find out whether there were any differences in the anatomy of cranium, which may contribute the pathogenesis or risk factors of CSDH.
Methods
The study population was consisted of 87 patients with CSDH and 100 patients with transient ischemic attack (TIA) from 2006 to 2013. We classified into four groups; group A (CSDH male 47), group B (CSDH female 40), group C (TIA male 50), and group D (TIA female 50). We measured the size of the cranium in the computed tomography scans, retrospectively. We define the difference of cranium (Dc), which is difference between the right and left radiuses.
A chronic subdural hematoma (CSDH) is a slowly growing encapsulated collection of blood and its breakdown products between the dura mater and the arachnoid.12) CSDH is a condition mostly present in older people. Men are more commonly affected than women. One rationale for male dominance could be that men generally have a greater exposure to injuries.10,17) Although men sustain nearly two to three times as many brain injuries as women,6) it could not enough to explain the reason for male predominance on CSDH.
It is well documented that mean brain size (weight or volume) is 9-12% larger in men than in women.16) There is evidence that some brain regions show age-associated volume decline and that men undergo more accelerated cerebral aging than women.8) The purpose of this study is to find out whether there were any differences in the anatomy of cranium, which may contribute the pathogenesis or risk factors of CSDH.
We treated 56 patients with CSDH by burr-hole drainage from 2011 to 2013. There were 47 males and 9 females (sex ratio 5.2:1). The mean age was 70 years old (range 43 to 86 years). The number of female patients was few to compare the anatomical difference according to the gender. We included consecutive 31 female patients with CSDH from 2006 to 2009. We also included consecutive 100 patients with transient ischemic attack (TIA) to compare the morphology and size of the cranium. These patients had been managed conservatively in this hospital between 2006 and 2013. The study population was consisted of 87 patients with CSDH and 100 patients with TIA. In all cases, the diagnosis was made by the cranial computed tomography (CT) scan. We excluded patient with conservatively treated CSDH, since differentiation of subdural hygroma from CSDH is often impossible by CT alone. We also excluded patients with TIA who had a history of surgical or medical treatment for brain disease.
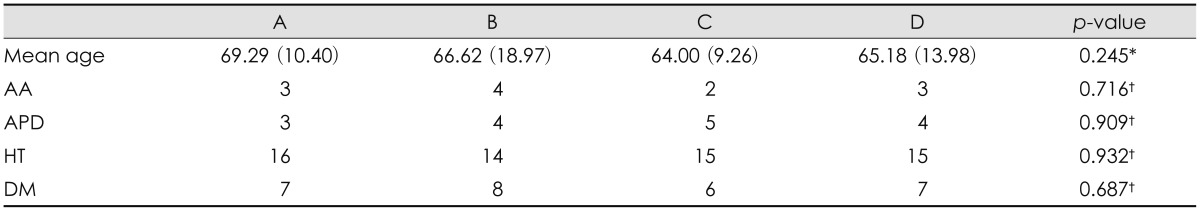
We classified into four groups; group A (CSDH male 47), group B (CSDH female 40), group C (TIA male 50), and group D (TIA female 50). We measured the size of the cranium in the CT scans, retrospectively. Demographic characteristics of these groups were presented in Table 1. There were no statistically significant differences.
First, we selected a CT axial image where the third ventricle was best visualized. Second, we drew a midline from the glabella to the inion. Finally, we measured the length of cranium from the glabella to the inion (Lap), the width of cranium (Wc), and the maximal radius from midline to the right (Rr) and left (Rl) ends (Figure 1). We obtained the ratio of cranium (Rc), dividing the width by the length (Wc/Lap) and difference of cranium (Dc), difference between the right and left radiuses. Although measurement of the exact cranial asymmetry requires reconstruction 3D program, gross asymmetry could be assured on the conventional CT axial image. Statistical analysis was performed with ANOVA and t-test. All p value less than 0.05 were considered statistically significant.
The average length and width of male cranium were larger than those of female cranium (Table 2). The radius of the left side was slightly larger than that of the right side in all groups except group A (CSDH male). Although the length and width of the male groups were larger than those of the female groups, the Rc was quite similar in all groups. However, the Dc was significantly higher in patients with CSDH (group A and B)(p=0.03).
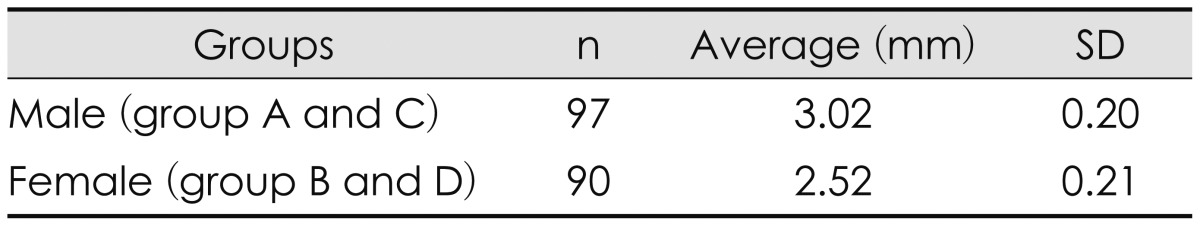
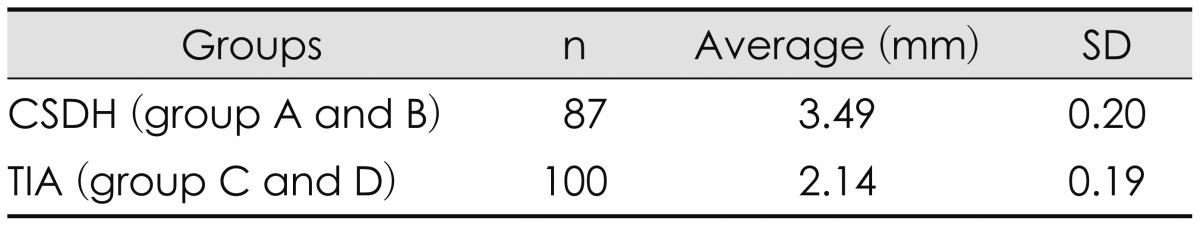
We also compared with gender (A+C vs. B+D) and disease (A+B vs. C+D) groups. The mean Dc was 3.02 mm in male group (group A and C) and 2.52 mm in female group (group B and D). Although the mean Dc of male group was larger than that of female group, this difference was not statistically significant (by t-test, p=0.24)(Table 3). The mean Dc was 3.49 mm in CSDH group (group A and B) and 2.14 mm in TIA group (group C and D). The mean Dc of CSDH group was significantly larger than that of TIA group (by t-test, p<0.05)(Table 4).
The average size of male cranium was larger than that of female cranium. The radius of the left side was usually larger than that of the right side due to cerebral dominance. Although the Rc was quite similar in all groups, the Dc was significantly higher in patients with CSDH either male or female.
CSDH occur in the dural border cell (DBC) layer, located between the dura mater and the arachnoid and the dissection of these layer creates a subdural cavity.9) Patients with extensive brain atrophy (elderly and alcoholics) or conditions resulting in intracranial hypotension (ventriculoperitoneal shunt) are vulnerable to developing CSDH.1) Traversing veins are being stretched by the shrinking brain until only a minor additional force is sufficient to cause the rupture of the bridging veins and create the hematoma.1) This is followed by fibrin deposition, organization, enzymatic fibrinolysis, and liquefaction of the clot. An inflammatory reaction occurs, and neomembranes (inner or visceral and outer or parietal membranes) are formatted with the growth of neocapillaries and enzymatic hyperfibrinolysis.15) CSDH tend to gradually enlarge because repeated micro-hemorrhage may lead to clinical signs and symptoms of increased intracranial pressure or compression brain structures.12)
The pathogenesis of CSDHs remains complex and hypothetical.14) Risk factors are not well understood yet, especially in elderly patients.2,3) Kim et al.11) assumed that medical history did not significantly influence the recurrence rate. They correlated to progressive brain atrophy with enlargement of the subdural space. In addition, the dural blood vessel increases the fragility, and the hemostasis inside the hematoma is not sufficient, because of the simultaneous activation of coagulation and fibrinolytic cascade, with an increased expression of tissue-type plasminogen activator.14) In general, advancing age has been associated with decreased brain tissue and increased cerebrospinal fluid (CSF). The major predisposing factor is that generalized cerebral atrophy and the venous fragility increase. Some reported that in patients over 50 years of age, the mass of the brain is reduced by approximately 200 g, which results in an increased extracerebral volume of up to 11%.13) So we want to validate a theory that the morphologic difference between CSDH and normal group is the aggravating factor of development of CSDH. If it became an authenticated case, it is helpful to study the pathogenesis of CSDH.
In the present study, we found a statistically significant difference in the maximal radius between CSDH female and TIA female group. It means that the asymmetry of women's cranium with CSDH is significantly higher than with TIA. We also found a statistically significant difference in the maximal radius between all patients with CSDH and all patients with TIA, regardless of the sex difference. It implies that the asymmetrical cranium may be a significant risk factor of CSDH. We could assume that the bulging-headed person is prone to develop a CSDH under trauma. We selected patients with TIA as a control group, since their age-distribution was similar.
Atrophy of the brain is an aging process. Although the brain atrophy is progressive, the cranium is constant. So the newly developed dead space after brain atrophy is gradually filled with CSF or others, according to Monro-Kelly's doctrine. The negative pressure from brain atrophy is constant on each side of the cranium, according to Boyle's law which the pressure of gas tried to hold at a constant temperature change by inverse with the volume of the gas.
PV=k; Where P is the pressure of gas, V is the volume of the gas, and k is a constant.
Since diffuse brain atrophy decreases the left and right hemispheres in the same ratio, the negative pressure of the both hemispheres is the same. As the degree of atrophy is constant, the decreased length of brain diameter on the large hemisphere is longer than on the small hemisphere. So, the distance from the skull to the cerebral cortex in the shrunken hemisphere is longer on the side of large hemisphere. The bridging vein is more stretched on the large hemisphere, so it may be tore by a trivial injury. If the distance exceeds the length of the arachnoid trabeculae, separation of the DBC layer will occur at the large hemisphere, which will create so-called the subdural space. This anatomical asymmetry of the cranium influences the left prevalence of CSDH. Separation of the DBC layer is easy in a large cranium with severe atrophy, which could explain the male prevalence. Anatomically, female is hard to develop CSDH unless the cranial asymmetry is great.
The gender differences in normal brain structure and function have been carried out.12) Male brain is larger than female brains in all locations, especially most prominent in frontal and occipital lobe, bilaterally.10) And the anterior regions of corpus callosum decrease in width at earlier age in men than women.8,10) Age-specific changes in brain size were significantly greater in men than women.5,7) With increasing the brain atrophy, the CSF in subdural space increase, so it is result in CSDH. Age-specific changes in brain size were significantly greater in men than women for CSF volume, the sylvian fissure CSF volume, and the parietooccipital region area.4) This can be another reason for the high prevalence of CSDH in males. The male prevalence is related to the cranial size and asymmetry along with great exposure of males to injury, estrogen effect on the capillaries, or fewer medical visit of females.10)
References
1. Baechli H, Nordmann A, Bucher HC, Gratzl O. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev. 2004; 27:263–266. PMID: 15148652.

2. Beatty RA. Subdural haematomas in the elderly: experience with treatment by trephine craniotomy and not closing the dura or replacing the bone plate. Br J Neurosurg. 1999; 13:60–64. PMID: 10492687.

3. Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000; 11:399–406. PMID: 10918008.

4. Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, et al. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998; 55:169–179. PMID: 9482358.
5. Cowell PE, Allen LS, Zalatimo NS, Denenberg VH. A developmental study of sex and age interactions in the human corpus callosum. Brain Res Dev Brain Res. 1992; 66:187–192. PMID: 1606684.

6. Ficker-Terill C, Flippo K, Antoinette T, Braunling-McMorrow D. Overview of brain injury. In : Lash M, McMorrow DB, Tyler J, Antoinette T, editors. Training manual for certified brain injury specialists (CBIS): Level 1 core competencies. McLean, VA: American Academy for the Certification of Brain Injury Specialists, Brain Injury Association of America;2004.
7. Giuffrè R, Palma E, Liccardo G, Sciarra F, Pastore FS, Concolino G. Sex steroid hormones in the pathogenesis of chronic subdural haematoma. Neurochirurgia (Stuttg). 1992; 35:103–107. PMID: 1508287.

8. Gur RC, Gunning-Dixon FM, Turetsky BI, Bilker WB, Gur RE. Brain region and sex differences in age association with brain volume: a quantitative MRI study of healthy young adults. Am J Geriatr Psychiatry. 2002; 10:72–80. PMID: 11790637.

9. Haines DE, Harkey HL, al-Mefty O. The "subdural" space: a new look at an outdated concept. Neurosurgery. 1993; 32:111–120. PMID: 8421539.
10. Kanat A, Kayaci S, Yazar U, Kazdal H, Terzi Y. Chronic subdural hematoma in adults: why does it occur more often in males than females? Influence of patient's sexual gender on occurrence. J Neurosurg Sci. 2010; 54:99–103. PMID: 21423076.
11. Kim SG, Yang JC, Kim TW, Park KH. Factors that influence to chronic subdural hematoma recurrence. Korean J Neurotrauma. 2013; 9:81–86.

12. Laffey PA, Peyster RG, Nathan R, Haskin ME, McGinley JA. Computed tomography and aging: results in a normal elderly population. Neuroradiology. 1984; 26:273–278. PMID: 6462433.

13. Misra M, Salazar JL, Bloom DM. Subdural-peritoneal shunt: treatment for bilateral chronic subdural hematoma. Surg Neurol. 1996; 46:378–383. PMID: 8876720.

14. Park SH, Kang DH, Park J, Hwang JH, Hwang SK, Sung JK, et al. Fibrinogen and D-dimer analysis of chronic subdural hematomas and computed tomography findings: a prospective study. Clin Neurol Neurosurg. 2011; 113:272–276. PMID: 21156338.

15. Sousa EB, Brandão LF, Tavares CB, Borges IB, Neto NG, Kessler IM. Epidemiological characteristics of 778 patients who underwent surgical drainage of chronic subdural hematomas in Brasília, Brazil. BMC Surg. 2013; 13:5. PMID: 23452673.

16. Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006; 129(pt 2):386–398. PMID: 16339797.

17. Wu X, Hu J, Zhuo L, Fu C, Hui G, Wang Y, et al. Epidemiology of traumatic brain injury in eastern China, 2004: a prospective large case study. J Trauma. 2008; 64:1313–1319. PMID: 18469656.

FIGURE 1
On the best image of well-visualized of the 3rd ventricle of axial computed tomography images of all patients, the midline was set from anterior falx and post falx and we measure the maximal radius on each side. Lap: length of cranium (from glabella to inion), Wc: width of cranium, Rr: radius of the right side, Rl: radius of the left side.
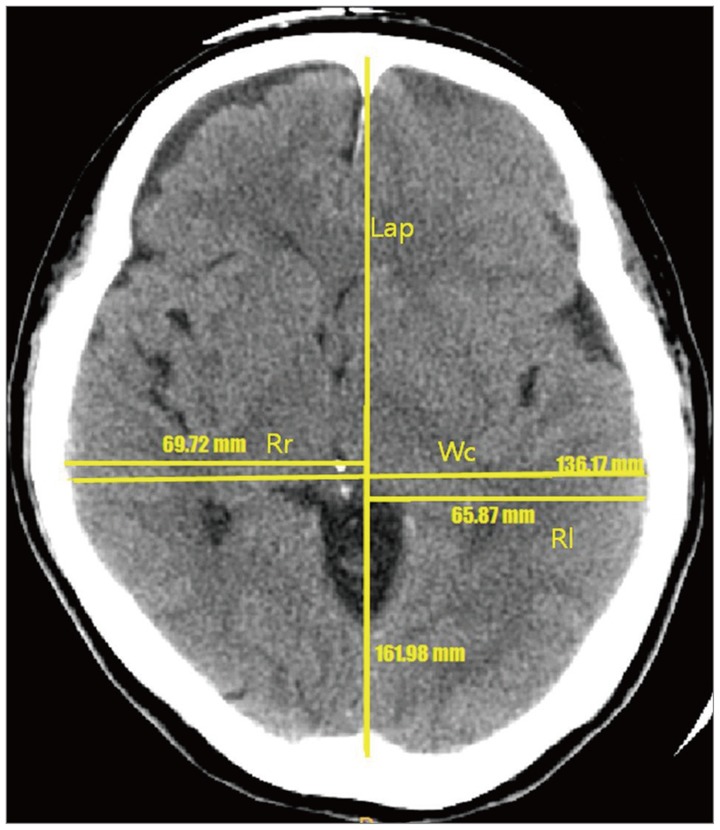
TABLE 2
The average value of cranial measurement in groups A, B, C, and D
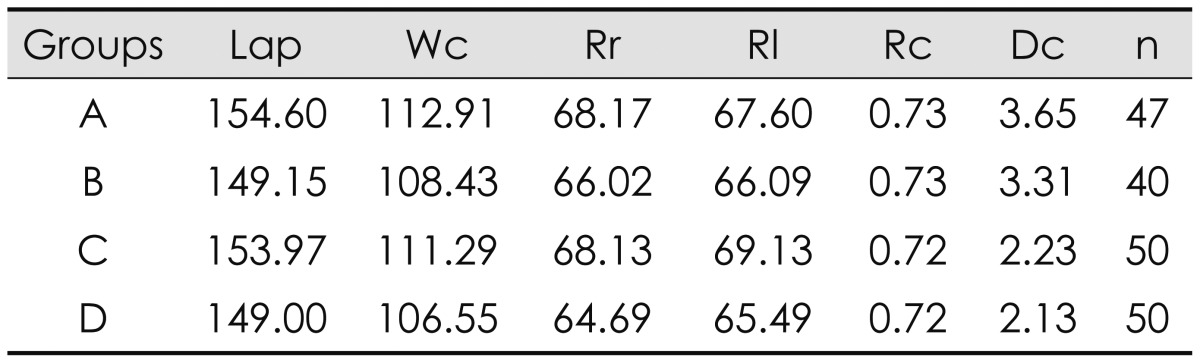
Group A: male chronic subdural hematoma, Group B: female chronic subdural hematoma, Group C: male transient ischemic attack, Group D: female transient ischemic attack. Lap: length of cranium (from glabella to inion), Wc: width of cranium, Rr: radius of the right side, Rl: radius of the left side, Rc: ratio of cranium (Wc/Lap), Dc: difference of cranium




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download