Abstract
Objective
Chronic subdural hematoma (CSDH) is a common intracranial hemorrhage that is associated with significant morbidity. Bilateral lesions are occasionally found in neurosurgical practice. The purpose of this study is to analyze clinical characteristics of bilateral CSDH compared with unilateral CSDH.
Methods
Between January 2005 and January 2013, the authors treated 114 surgical patients with CSDH. Clinical presentations, precipitating factors, computed tomography (CT) findings, postoperative complications, and outcomes of patients were retrospectively analyzed in the bilateral and unilateral CSDH groups.
Results
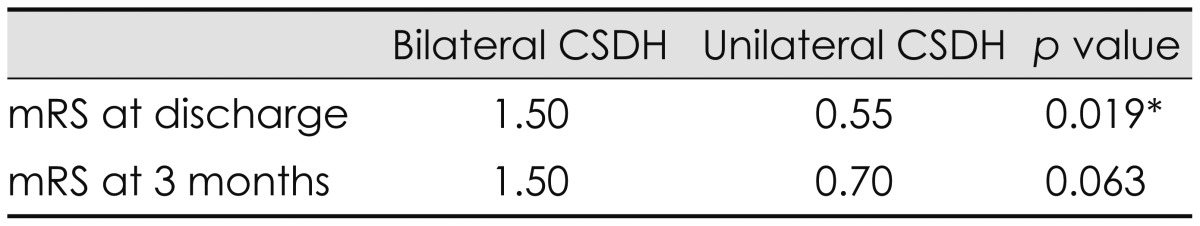
Bilateral CSDH was identified in 28 (24.6%) of the 114 CSDH patients. The mean age was 77.85 years in the bilateral CSDH group. The frequency of altered consciousness as a presenting symptom was significantly higher in the bilateral CSDH, and that of hemiparesis was significantly higher in the unilateral CSDH (p=0.015). Diabetes mellitus was more common in the bilateral CSDH (p=0.001). CT scans revealed significant differences in the degree of midline shift (p=0.001). The mean modified Rankin scale at discharge was 1.5 in the bilateral CSDH group and 0.6 in the unilateral group (p=0.019).
Conclusion
Bilateral CSDH showed different clinical characteristics from unilateral CSDH. Bilateral CSDH is prone to occurrence in the patient of old and diabetics. The patients of bilateral CSDH seem to reveal worse mental status and neurologic sign than unilateral CSDH in both baseline and postoperative state.
Chronic subdural hematoma (CSDH) is a common form of intracranial hemorrhage that correlate significantly with morbidity.11,18,33) Management of CSDH is non-surgical or surgical. Neurosurgeons have performed various surgical techniques, such as burr-hole craniotomy with or without continuous closed-system drainage,2,16,17,21) small craniotomy with endoscopic removal,23) and large craniotomy followed by, hematoma removal with membranectomy.8) The prognosis after surgery is usually good because of development of modern imaging methods, and improvement in surgical techniques.32)
Unilateral CSDH occurs in the majority of patients, but bilateral CSDH is occasionally found in neurosurgical practice. The overall incidence of bilateral CSDH has been reported to vary from 16% to 20%.4,20,22) Kurokawa et al.13) reported bilateral CSDH cases should be treated with simultaneous decompression of bilateral hematoma pressure as early as possible. The recurrence rate has been variably reported to be significantly higher in patients with bilateral CSDH than in those with unilateral CSDH.14,20) Another study has reported that bilateral CSDH is an independent predictor of CSDH recurrence.27) Although bilateral CSDH has been studied in previous studies, there are few studies comparing the clinical characteristics of bilateral and unilateral CSDH cases. The purpose of this study was to analyze clinical presentations, precipitating factors, computed tomography (CT) findings, postoperative complications, and outcomes of bilateral CSDH compared with unilateral CSDH.
A total of 131 patients with CSDH who were treated at Dongguk University Ilsan Hospital from January 2005 through January 2013 were enrolled in this study. This study excluded patients with 1) concomitant traumatic brain injury (n=3), 2) CSDH due to complications of prior neurosurgical procedures (n=10), such as cerebrospinal fluid shunting or craniotomy, and 3) conservative treatment without surgery (n=4). Finally, 114 patients were included in the study and were divided into the bilateral and unilateral CSDH groups.
Clinical presentations of the 114 patients were documented at the emergency department or the outpatient clinic. Presenting symptoms included headache, dizziness, altered consciousness, hemiparesis, dysarthria, gait disturbance, and seizure.
All patients underwent brain CT scan or magnetic resonance imaging (MRI) immediately after arrival at the emergency department or the outpatient clinic, and CSDH was confirmed by CT and MRI. Brain CT scans were interpreted on the basis of the following findings: 1) the thickness of hematoma measured at the maximal diameter; 2) the location of hematoma (unilateral or bilateral cerebral convexity); 3) midline shift as measured by using deviation of the septum pellucidum from the central position; and 4) Hounsfield units of hematoma at the site of the highest density (hypodense, isodense, hyperdense, or mixed). All patients underwent drilling of burr holes on the side of the subdural hematoma and irrigation of the subdural space with normal saline. All patients had closed-system drainage, with the aid of a silicone tube indwelled in the subdural space and tunneled under the scalp to the exit point of the incision line. The drains were removed when drainage had decreased or stopped. The drainage of all patients had kept until postoperative days 3.
Local recurrence was defined as recurrence of CSDH that required reoperation due to clinical and radiological aggravation. The radiological aggravation consisted of an increase in the thickness of hematoma on follow-up CT scans within 3 months postoperatively.
Modified Rankin scale (mRS) scores were measured by the neurosurgeons when patients were discharged from the hospital and revisited the outpatient clinic within 3 months postoperatively. We assessed the outcomes of CSDH using the mRS at discharge and 3 months postoperatively. Postoperative complications included acute subdural hemorrhage, infection, new-onset seizure, pneumocephalus, and hydrocephalus.
All patients were divided into 2 groups: the bilateral and unilateral CSDH groups. This study is of retrospective design, and it analyzed data, including clinical presentations, precipitating factors, CT findings, postoperative complications, and outcomes. Statistical analysis was performed using SPSS statistical software package 18.0 (SPSS Inc., Chicago, IL, USA). Comparisons between the 2 groups were made by using the independent samples t-test and crosstabs analysis. Statistical significance was set at p<0.05.
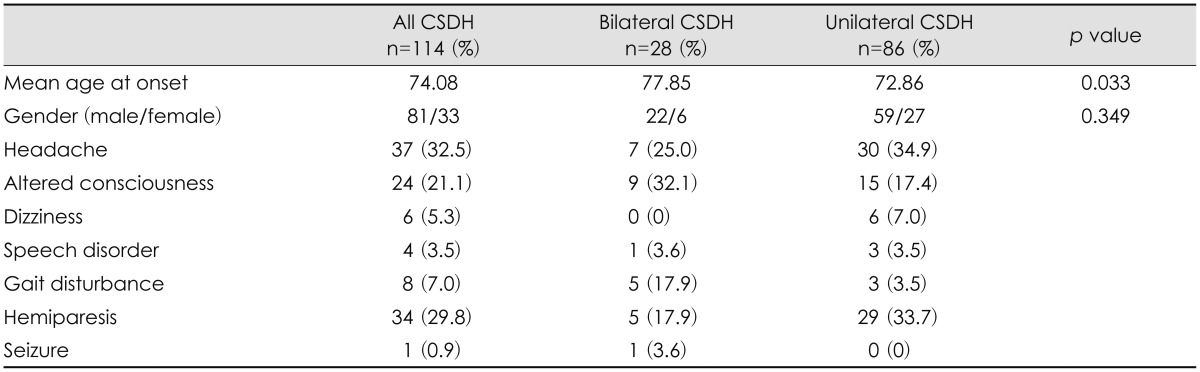
The mean age of the patients was 74.08 years. The mean age was 77.85 years in the bilateral CSDH group and 72.86 years in the unilateral CSDH group (p=0.033). The mean age is older in the bilateral CSDH group than in the unilateral CSDH group. Overall, there was a male predominance (81 patients, 71.1%). The female/male ratios were 6:22 in the bilateral CSDH group and 27:59 in the unilateral CSDH group. No significant difference in sex was found between the 2 groups.
The characteristics and clinical presentations of the patients in both groups are shown in Table 1. The most common symptom was altered consciousness in the bilateral CSDH group and headache in the unilateral CSDH group. Other clinical presentations included gait disturbance, dizziness, hemiparesis, speech disorder, and seizure. Altered consciousness and gait disturbance were significantly more common in the bilateral CSDH group than in the unilateral CSDH group (p=0.015). Motor deficits were more commonly presented in the unilateral CSDH group (p=0.015).
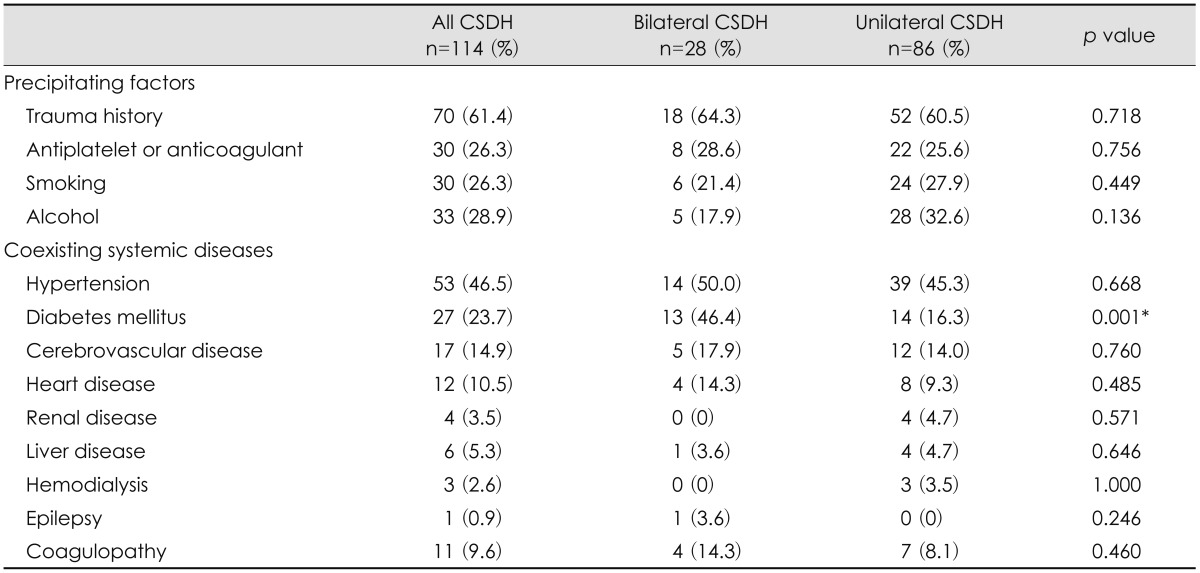
The precipitating factors of the patients in both groups are shown in Table 2. History of head injury was present in 64.3% of patients in the bilateral CSDH group and 60.5% of patients in the unilateral CSDH group. No significant difference in trauma history was found between the 2 groups. History of diabetes mellitus was observed in 13 patients (46.4%) in the bilateral CSDH group and 14 patients in the unilateral CSDH group (16.3%)(p=0.001). Other precipitating factors and concurrent systemic diseases of CSDH were hypertension, history of smoking or alcohol intake, cerebrovascular disease, heart disease, renal disease, and liver disease. They were not significantly different between the 2 groups.
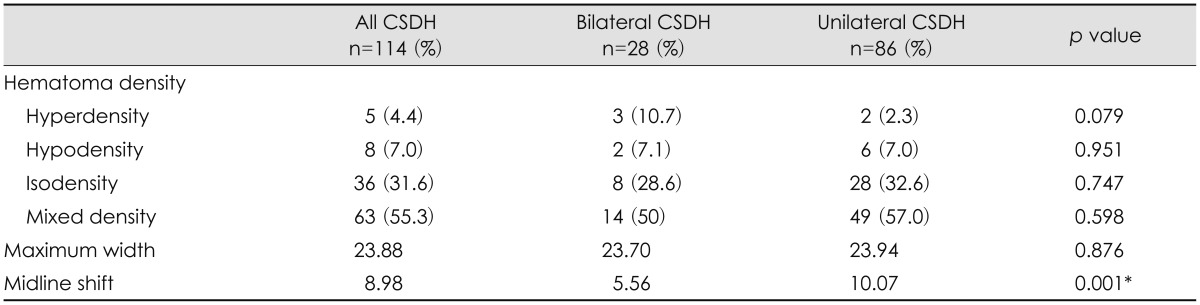
The density of hematoma on CT scans was categorized into hypodensity, hyperdensity, isodensity, or mixed density (Table 3). No significant difference was found in the density of hematoma on CT scans between the 2 groups. The mean degree of midline shift on CT scans was a significantly greater in the unilateral CSDH group than in the bilateral CSDH group (p=0.001).
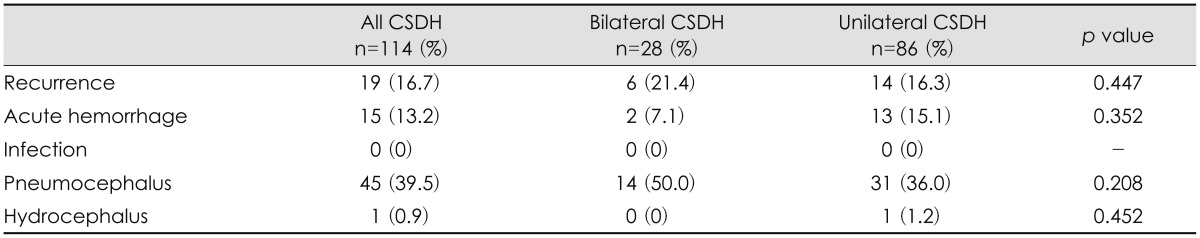
The postoperative complications and outcomes of the patients in the 2 groups are shown in Table 4. Recurrence was found in 6 patients (21.4%) in the bilateral CSDH group and 14 patients (16.3%) in the unilateral CSDH group, but the difference was not statistically significant. Pneumocephalus was the most common postoperative complication in all patients. All postoperative complications showed no significant differences between the 2 groups. Overall favorable outcome (mRS 0-2) was achieved in 85.9% of patients in all patients, 67.8% of patients in the bilateral CSDH group, and 91.8% of patients in the unilateral CSDH group. The mean mRS at discharge was 1.5 in the bilateral CSDH group and 0.6 in the unilateral CSDH group (p=0.019). The mean mRS at the outpatient clinic within 3 months postoperatively was 0.89 in all patients, 1.5 in the bilateral CSDH group, and 0.7 in the unilateral CSDH group (p=0.063). Most patients had a good postoperative outcome after surgical treatment. One patient who belonged to unilateral group died of pneumonia during treatment (Table 5).
The incidence of bilateral CSDH was 24.8% in this study. This is close to the incidences of previous studies that have been reported to vary from 16% to 20%.4,20,22) The mean age of CSDH patients in this study was 74 years. Spallone et al.26) have reported that bilateral CSDH tends to occur in patients older than 75 years. In this study, the mean age of the patients was 77 years in the bilateral CSDH and 72 years in the unilateral CSDH group. The mean age was older in the bilateral CSDH group than in the unilateral CDSH group (p=0.033). CSDH is more common in elderly subjects because of age-related decreases in brain volume and increases in venous fragility.1,26) This increases tension on the cerebral bridge veins, and the greater movement of the brain within the cranium makes these veins vulnerable to trauma.5,28) Thus, we thought that elderly subjects were more likely to develop bilateral CSDH. CSDH predominantly occurred in males, with no significant difference between the 2 groups.
In this study, headache is the most common symptom in all patients. The incidence of headache varies among different studies, ranging from 14% to 80%.6,10) Adhiyaman et al.1) have shown that the most common presentation in elderly subjects (50-70%) is altered consciousness which manifests as varying degrees of confusion, drowsiness, or coma. Tsai et al.29) have suggested that bilateral CSDH has symptoms of raised intracranial pressure more frequently than unilateral CSDH. In our study, altered consciousness was the most common presentation and more common in the bilateral CSDH group than in the unilateral CSDH group. Some patients in the bilateral CSDH group presented stupor or coma. Hemiparesis occurred less frequently in the bilateral CSDH group. Okuyama et al.19) measure cerebral blood flow (CBF) in 34 patients with bilateral CSDH and the CBF reductions are showed in the frontal, parietal and occipital cortices in the thin hematoma side, and in the putamen in the thick hematoma side in the brain shifted group. Huang et al.9) suggested that the shifted force of CSDH is accompanied by a secondary CBF reduction in the deep cerebral regions which is a major cause of neurological dysfunction and patients with a bilateral CSDH have less opportunity for central brain structures to deviate owing to counterbalance of the mass effect on both sides. We found that the mean degree of midline shift on CT scans was a significantly greater in the unilateral CSDH and supposed that shifted force was easily accompanied in unilateral SDH group. This is correlated with bilateral CSDH had a lower incidence of hemiparesis.
Rust et al.25) have indicated that anticoagulant therapy can be used in a significant percentage of patients with CSDH. Tsai et al.29) have reported that the frequency of anticoagulant and antiplatelet use is significantly higher in the bilateral CSDH group than in the unilateral CSDH group. In this study, however, the frequency of taking antiplatelets or anticoagulants showed no significant difference between the 2 groups. Kim et al.12) have demonstrated that the presence of diabetes mellitus is significantly related to poor prognosis. Cheung et al.3) have reported that history of diabetes mellitus is the only patient characteristic that markedly increases the risk of intracranial hemorrhage in surgically treated patients. Diabetes mellitus is also a significant predictor of mortality after moderate to severe traumatic brain injury.15) The pathophysiology of CSDH has not yet been fully elucidated. Some studies have reported that increased permeability of the capillaries in the outer membrane of hematoma can cause the enlargement of CSDH.7,34) Vascular permeability activates the kallikrein system which may cause blood extravasation and plasma exudation.
Fujisawa et al.7) have shown that the kallikrein system is activated histologically and biochemically in the outer membrane of hematoma. Increased capillary permeability is an early phenomenon in diabetes mellitus that affects the microvasculature of the brain and many other organs.31) Clinical and experimental evidence suggests that hyperglycemia may impair autoregulation and increase permeability in microvessels.35) This leads us to assume that patients with diabetic mellitus are likely to develop bilateral CSDH rather than unilateral CSDH in spite of the same traumatic stress to the head.
Most of the CSDH patients have good outcomes after surgical treatment.1,26) Surgical treatment of CSDH improves neurological recovery and radiologic findings. In previous studies, postoperative outcomes were no significant differences between the bilateral and unilateral CSDH groups.9) Whereas in this study they were poorer in the bilateral CSDH group. Van Havenbergh et al.30) have reported that the only statistically significant factor for clinical outcomes is the neurological condition at the time of treatment. Rozzelle et al.24) have documented that patients with Glasgow coma scale under 7 on admission have high mortality rates. In our study, the initial neurological state was poor in the bilateral CSDH group compared to the unilateral CSDH group. Furthermore, in the bilateral CSDH group, some patients were in a very poor neurologic state that included coma or stupor at the time of treatment and showed a poor outcomes at discharge (mRS 4-6). Huang et al.9) have suggested that patients with bilateral CSDH may present with vague symptoms found incidentally during workup. Therefore, a delayed or missed diagnosis of CSDH is more common in patients with bilateral CSDH. Bilateral CSDH cases show rapid and progressive aggravation, and thus they should be treated as early as possible, even if they show minimal neurologic deficits.17)
The limitation of this study was its retrospective design. In this study, data was collected through medical records and imaging reviews and thus less accurate than that in prospective studies. The duration of evaluation of neurological outcomes was only within 3 months postoperatively, and follow-up information was difficult to obtain in cases of no revisit to the outpatient clinic. Patients receiving conservative treatment without surgery were excluded from this study. This may have influenced our results and have caused a selection bias. We did not analyze correlations between prognosis and HbA1c which shows the average level of blood glucose. This correlation will demonstrate that poor diabetes mellitus control can cause bilateral CSDH.
Bilateral CSDH showed different clinical characteristics from unilateral CSDH. Bilateral CSDH is prone to occurrence in the patient of old and diabetics. The patients of bilateral CSDH seem to reveal worse mental status and neurologic sign than unilateral CSDH in both baseline and postoperative state.
References
1. Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural haematoma in the elderly. Postgrad Med J. 2002; 78:71–75. PMID: 11807186.
2. Camel M, Grubb RL Jr. Treatment of chronic subdural hematoma by twist-drill craniotomy with continuous catheter drainage. J Neurosurg. 1986; 65:183–187. PMID: 3723175.
3. Cheung RT, Eliasziw M, Meldrum HE, Fox AJ, Barnett HJ. North American Symptomatic Carotid Endarterectomy Trial Group. Risk, types, and severity of intracranial hemorrhage in patients with symptomatic carotid artery stenosis. Stroke. 2003; 34:1847–1851. PMID: 12829862.

4. De Jesús O, Pacheco H, Negron B. Chronic and subacute subdural hematoma in the adult population. The Puerto Rico experience. P R Health Sci J. 1998; 17:227–233. PMID: 9883468.
5. Ellis GL. Subdural hematoma in the elderly. Emerg Med Clin North Am. 1990; 8:281–294. PMID: 2187683.

6. Fogelholm R, Heiskanen O, Waltimo O. Chronic subdural hematoma in adults. Influence of patient's age on symptoms, signs, and thickness of hematoma. J Neurosurg. 1975; 42:43–46. PMID: 1167376.
7. Fujisawa H, Ito H, Kashiwagi S, Nomura S, Toyosawa M. Kallikrein-kinin system in chronic subdural haematomas: its roles in vascular permeability and regulation of fibrinolysis and coagulation. J Neurol Neurosurg Psychiatry. 1995; 59:388–394. PMID: 7561918.

8. Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993; 33:67–72. PMID: 8355849.
9. Huang YH, Yang KY, Lee TC, Liao CC. Bilateral chronic subdural hematoma: what is the clinical significance? Int J Surg. 2013; 11:544–548. PMID: 23707986.

10. Jones S, Kafetz K. A prospective study of chronic subdural haematomas in elderly patients. Age Ageing. 1999; 28:519–521. PMID: 10604502.

11. Kang HL, Shin HS, Kim TH, Hwang YS, Park SK. Clinical analysis of recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2006; 40:262–266.
12. Kim YI, Lee JH, Park SW, Nam TK, Min BK, Hwang SN. Analysis of prognostic factors for chronic subdural hematoma. J Korean Neurotraumatol Soc. 2008; 4:14–18.

13. Kurokawa Y, Ishizaki E, Inaba K. Bilateral chronic subdural hematoma cases showing rapid and progressive aggravation. Surg Neurol. 2005; 64:444–449. discussion 449. PMID: 16253697.

14. Lee SC, Kang JK, Jung HT, Dho JO. Factors affecting brain re-expansion after simple burr hole drainage in chronic subdural hematoma. J Korean Neurosurg Soc. 1998; 27:757–762.
15. Ley EJ, Srour MK, Clond MA, Barnajian M, Tillou A, Mirocha J, et al. Diabetic patients with traumatic brain injury: insulin deficiency is associated with increased mortality. J Trauma. 2011; 70:1141–1144. PMID: 21610428.

16. Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981; 54:637–645. PMID: 7014792.

17. Markwalder TM, Seiler RW. Chronic subdural hematomas: to drain or not to drain? Neurosurgery. 1985; 16:185–188. PMID: 3974829.

18. Oishi M, Toyama M, Tamatani S, Kitazawa T, Saito M. Clinical factors of recurrent chronic subdural hematoma. Neurol Med Chir (Tokyo). 2001; 41:382–386. PMID: 11561348.

19. Okuyama T, Saito K, Fukuyama K, Yamamoto K, Morimoto M, Aburano T. [Clinical study of cerebral blood flow in bilateral chronic subdural hematoma measured by 99mTc-HMPAO SPECT]. No To Shinkei. 2000; 52:709–714. PMID: 11002481.
20. Penchet G, Loiseau H, Castel JP. [Chronic bilateral subdural hematomas]. Neurochirurgie. 1998; 44:247–252. PMID: 9864695.
21. Reinges MH, Hasselberg I, Rohde V, Küker W, Gilsbach JM. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. J Neurol Neurosurg Psychiatry. 2000; 69:40–47. PMID: 10864602.

22. Robinson RG. Chronic subdural hematoma: surgical management in 133 patients. J Neurosurg. 1984; 61:263–268. PMID: 6737050.

23. Rodziewicz GS, Chuang WC. Endoscopic removal of organized chronic subdural hematoma. Surg Neurol. 1995; 43:569–572. discussion 572-573. PMID: 7482236.

24. Rozzelle CJ, Wofford JL, Branch CL. Predictors of hospital mortality in older patients with subdural hematoma. J Am Geriatr Soc. 1995; 43:240–244. PMID: 7884110.

25. Rust T, Kiemer N, Erasmus A. Chronic subdural haematomas and anticoagulation or anti-thrombotic therapy. J Clin Neurosci. 2006; 13:823–827. PMID: 16997707.

26. Spallone A, Giuffrè R, Gagliardi FM, Vagnozzi R. Chronic subdural hematoma in extremely aged patients. Eur Neurol. 1989; 29:18–22. PMID: 2707288.

27. Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008; 63:1125–1129. discussion 1129. PMID: 19008766.
28. Traynelis VC. Chronic subdural hematoma in the elderly. Clin Geriatr Med. 1991; 7:583–598. PMID: 1868412.

29. Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF. A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: precipitating factors and postoperative outcomes. J Trauma. 2010; 68:571–575. PMID: 20065879.

30. van Havenbergh T, van Calenbergh F, Goffin J, Plets C. Outcome of chronic subdural haematoma: analysis of prognostic factors. Br J Neurosurg. 1996; 10:35–39. PMID: 8672256.
31. Viberti GC. Increased capillary permeability in diabetes mellitus and its relationship to microvascular angiopathy. Am J Med. 1983; 75:81–84. PMID: 6673594.

32. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003; 74:937–943. PMID: 12810784.

33. Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003; 98:1217–1221. PMID: 12816267.

34. Yamashima T, Yamamoto S, Friede RL. The role of endothelial gap junctions in the enlargement of chronic subdural hematomas. J Neurosurg. 1983; 59:298–303. PMID: 6864298.

35. Yuan SY, Breslin JW, Perrin R, Gaudreault N, Guo M, Kargozaran H, et al. Microvascular permeability in diabetes and insulin resistance. Microcirculation. 2007; 14:363–373. PMID: 17613808.

TABLE 2
Precipitating factors and coexisting systemic diseases in 114 patients with chronic subdural hematoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download