Introduction
Neisseria cinerea (N. cinerea) is known as a non-pathogenic microorganism. It colonizes in the oropharynx, upper respiratory tract, and less commonly in the genital tract of healthy subjects [1234]. N. cinerea rarely causes infective diseases. Nevertheless, a variety of diseases caused by N. cinerea have been reported as follows; ophthalmia neonatorum [2], nosocomial pneumonia [3], recurrent bacterial peritonitis in peritoneal dialysis patient [5], proctitis [6], endocarditis [7], meningitis and septicemia [8]. However, in Korea, cases of acute septic arthritis and skin abscess [9], and meningitis [10] caused by N. cinerea were reported. A case of endocarditis caused by N. cinerea has not yet reported in Korea. We therefore report an experience of unusual case of endocarditis by N. cinerea and successful treatment of it. This is a case of infective endocarditis by N. cinerea in a 7-year-old Korean girl.
Case
A 7-year-old female patient was presented to our emergency department because of a 2-week history of fever, with body temperature up to 40.0℃ and general weakness. She had been diagnosed with tetralogy of Fallot and had undergone surgical repair in October 2006.
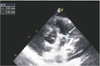
Pulmonary prosthetic valve replacement with Hancock II 23 mm valve was performed in January 2014 because of severe pulmonary regurgitation and moderate right ventricle dilatation on post-operation follow-up echocardiography. She did not present with coughing, sputum and rhinorrhea on admission. She looked ill, but her mental state was alert. During physical examination on the day of admission, her breathing sounds were clear, without rales or wheezing in either lung fields. Grade 2 or 3 systolic ejection murmur was heard at the left sternal border. No specific manifestations of infective endocarditis, such as splinter on nails or Janeway lesions on hands and feet, and Osler's nodes, were observed. No lymphadenopathy, splenomegaly or hepatomegaly was observed either. The initial laboratory testing revealed as follows; leukocytes of 12,230/mm3, hemoglobin 11.1 g/dL, hematocrit 31.8%, and platelet count 227,000/mm3. C-reactive protein increased up to 3.84 mg/dL. Liver function tests revealed normal levels of aspartate aminotransferase (20 IU/L) and alanine aminotransferase (12 IU/L). The chest radiography performed upon admission revealed no signs of active pulmonary lesions. We performed echocardiography on the day of admission and detected a mass-like vegetation approximately 9×4 mm in diameter at the pulmonary valve and a thickened pulmonary valve leaflet (Fig. 1). Blood cultures at 3 different area were conducted before initiation of antibiotics therapy. We started treatment with intravenous ceftriaxone (100 mg/kg/day), which is commonly used for endocarditis, on the day of admission and added gentamicin (6 mg/kg/day) from the seventh to the 11th day of admission.
N. cinerea was found in the blood cultures on admission. The guideline for minimal inhibitory concentration of N. cinerea has not been reported yet, so we started to administer intravenous ceftriaxone empirically. No bacteria were found in the blood cultures on the third day of admission. Despite that no bacteria were found in the blood cultures and treatment with antibiotics was administered, the fever persisted from the first to the 11th day of admission, and she complained about coughing, sputum, mild muscle aches, and general weakness. We decided to do further workup for pneumonia and perform chest CT for a suspicion of distal pulmonary embolism of the vegetation. On the chest radiography, haziness was observed in the right lower lobe area and on the basis of the laboratory findings, we checked a 4-fold increase in immunoglobulin G antibodies titer against Mycoplasma pneumoniae. Therefore, she was diagnosed as having mycoplasma pneumonia, so we administered oral clarithromycin (15 mg/kg/day) twice daily for 2 weeks. Her chest CT on the 11th day of admission demonstrated a suspected embolism in laterobasal segmental branch of the right lower pulmonary artery and patchy consolidation in right lower lung, which are suggestive of pneumonia (Fig. 2). We changed ceftriaxone and gentamicin to cefotaxime (200 mg/kg/day) on the 11th day of admission. However, a high fever of up to >39℃ persisted 2 or 3 times a day, and the value of C-reactive protein increased to up to 17.1 mg/dL, even though bacteria were not detected in the blood cultures. Therefore, we had a consultation with the division of infectious diseases on the 28th day of admission and changed to single injection of intravenous ceftriaxone (100 mg/kg/day). Thereafter, her fever gradually subsided, and she was well without symptoms or signs suggestive of endocarditis from the 30th day of admission.
The laboratory findings on the 30th day of admission were:
leukocytes 5,770/mm3, hemoglobin 9.9 g/dL, hematocrit 30.3%, and platelet count 445,000/mm3. C-reactive protein was 2.51 mg/dL.
She was discharged on 46th day of hospitalization with a prescription of oral cefdinir (14 mg/kg/day), for 7 days after discharge.
Discussion
N. cinerea was first recognized in 1906 in Europe. It frequently colonizes in the oropharynx, genital areas, and the upper respiratory tract. This microbe is known as a non-pathogenic strain that does not usually cause disease [1234]. Some cases caused by N. cinerea have been reported however, including ophthalmia neonatorum [2], nosocomial pneumonia [3], bacterial peritonitis [5], proctitis [6], tricuspid valve endocarditis [7], meningitis [810], acute septic arthritis and skin abscess [9].
Generally, viridian-type streptococci and Staphylococcus aureus are known as the most common causative organisms of infective endocarditis. Endocarditis caused by the viridian group of streptococci is most common after dental procedures, and fungal organisms can be encountered after cardiac surgery, especially in immunosuppressed patients. As implanted mechanical devices have a possibility to serve as the adhesive substrate for infection, those with prosthetic cardiac valve are most susceptible to endocarditis [11].
On echocardiography at the day of admission, we confirmed a mass-like vegetation around the pulmonary prosthetic valve, and N. cinerea was detected in the blood cultures. Although the route of N. cinerea infection was not clear, our patient underwent pulmonary prosthetic valve replacement about 6 months ago and had a dental care few weeks ago before developing fever. Therefore, we determined that N. cinerea which colonize in the oropharynx caused infective endocarditis.
N. cinerea was confirmed in the blood cultures, and we assessed the susceptibility of N. cinerea to penicillin, third-generation cephalosporin, tetracycline, and so forth in most of cases [4]. Benes et al. [7] reported a case of endocarditis caused by N. cinerea and tried to treat it with ampicillin because it showed susceptibility to it. However, response of treatment was poor, so they changed ampicillin to ceftriaxone and achieved good response.
On the basis of these case reports, we decided to continue the intravenous ceftriaxone. In our patient, bacteria were not detected in the blood cultures after the administration of antibiotics. However, her fever persisted with coughing and sputum; therefore, pneumonia was suspected. As a result of the workup for pneumonia, as mycoplasma pneumonia was superimposed on infective endocarditis, we added oral clarithromycin, and her general condition improved gradually.
However, mild fever persisted until the 28th day of admission and C-reactive protein increased up to 17.1 mg/L; therefore, we requested a consultation with the division of infectious diseases again, and changed cefotaxime and gentamicin to ceftriaxone alone.
Thereafter, her fever subsided from the 30th day of admission, and her C-reactive protein level tended to decrease. We determined that the cause of the lengthy fever may be the superimposition of mycoplasma pneumonia and concluded that the third-generation cephalosporin induced the good therapeutic response of the endocarditis caused by N. cinerea .
Prosthetic valve endocarditis (PVE) is the most severe form of infective endocarditis and is known to be closely related to serious complication with significant morbidity and mortality. PVE is divided into 2 types. Early PVE, which is onset within 60 days after valve replacement, and late PVE occurs more than two months after valve replacement. The incidence of PVE is approximately 2.5% among patients with valve replacement. It is known that staphylococci are the most common causative microorganism of PVE. All patients of these studies received surgical procedure and underwent intravenous antibiotics postoperatively [1213].
McElhinney et al. [14] reported their experience with infective endocarditis among patients who underwent transcatheter pulmonary valve replacement with Melody valve. As is well known, viridian-type streptococci and Staphylococcus aureus are the most common pathogenic organisms. All the patients in their study were treated with antibiotics, some of whom were surgically explanted with valves or received a second percutaneous pulmonary valve replacement.
In some cases of intractable endocarditis associated with valve regurgitation, obstruction, or fistula formation, surgical treatment should be considered [11]. However in our case of infective endocarditis, although the fever continued for a long time, she was hemodynamically stable. She was treated successfully with antibiotics.
This report is about a case of infective endocarditis caused by N. cinerea in a patient with pulmonary prosthetic valve. To our knowledge, endocarditis caused by N. cinerea has not been reported in Korea, so we report this as the first case in Korea, along with a literature review.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download