Introduction
Uterine leiomyomas affect approximately one fourth to one third of women in the United States, with 140,000~180,000 new diagnoses annually [1]. Patients complain of various symptoms including menorrhagia, metrorrhagia, localized cyclical pelvic pain, and pelvic fullness [1]. Uterine arterial embolization (UAE) has been accepted as an effective treatment alternative for symptomatic uterine fibroid [2-5]. Clinical failure has been also reported from 4% to 19% [2-6] and it has been suggested of technical or anatomical origin [7-10]. Anastomoses between the ovarian artery and uterine arteries have been regarded as a cause of early treatment failure as well as premature ovarian failure [9-12]. Magnetic resonance imaging (MRI) feature has been successfully used to assess uterine leiomyomas in symptomatic patients [13-15] and a recent report suggests that gadolinium (Gd)-diethylenetriaminepenta acetic acid (DTPA)-enhanced MRI can be used to identify the early treatment failure after UAE [16].
The objective of this study was therefore to assess the early treatment failure rate in symptomatic uterine fibroid patients with or without anastomotic collaterals between ovarian and uterine artery using Gd-DTPA enhanced MRI.
Methods
Anastomoses ovarian artery and uterine artery were recorded after reviewing pelvic angiogram and selective uterine angiogram in 169 consecutive women who underwent uterine fibroid treatment as a primary treatment for symptomatic uterine fibroids from November 1997 through August 2005. The mean patient age was 42.8 years (range, 25 to 57 years). The patients showed menorrhagia (n=27, 16%), pelvic pain (n=5, 3%), bulkrelated symptoms (n=8, 5%), or combination of the above symptoms (n=129, 76%). The mean numbers of leiomyomas were 3.6 (range, 1 to 11). Pre-procedural workup included full gynecologic evaluation by a gynecologist, routine history and physical examination, serum blood urea nitrogen and creatinine levels. All these women underwent ultrasonogram or pelvic MRI before UAE to confirm the presence of leiomyomas and exclude other pathology. Fibroids were determined to be the cause of symptoms in all patients. Women had other uterine pathology such as adenomyosis, infarcted leiomyomas, or other nonuterine disease were excluded. As a part of conventional follow-up, they were evaluated in clinical setting at 1~6 and 12 months and annually thereafter.
1. Angiographic procedure and UAE technique
All patients were counseled for the risk, benefits, and alternatives of uterine fibroid embolization, and written informed consent was obtained. Vascular access was done via the right or left common femoral artery. Initial abdominal aortography was done after placement of a standard 5-French (Fr) introducer sheath and a pigtail catheter at the level of the renal arteries. Then selective uterine angiography was performed by injection of 1~3 mL contrast material (Ultravist®, Bayer Healthcare, Wayne, NJ, USA) per second for 5 seconds using either a 4-Fr catheter (Barenstein Glide, Meditech, Natick, MA, USA) or another uterine catheter (Roberts Uterine Curve Catheter, Cook, Bloomington, IN, USA). The operator chose the rate and volume of injection according to the size of the uterine artery. If the catheter was judged to induce vasospasm, a microcatheter (Masstransit, Cordis Endovascular, Miami, FL, USA) was introduced into the uterine artery in a coaxial fashion and the angiographic catheter was pulled back into the internal iliac artery. The uterine artery was embolized to complete stasis of flow in the main uterine artery by using either 355~500 µm polyvinyl alcohol particles (Truefill, Cordis Endovascular, Miami, FL, USA) or 500~700 µm triacryl gelatin microspheres (Embosphereum, BioSphere Medical, Rockland, MA, USA). Repeat pelvic angiography with the pigtail catheter at the level of the renal arteries was then performed. If the ovarian artery was seen on the completion angiogram, it was investigated further with selective angiography. The endpoint for embolization was stasis of flow in the proximal uterine artery on angiographic imaging. Pre- and post-embolization arteriography were used to establish successful bilateral uterine artery occlusion.
2. Angiographic evaluation
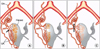
Angiographic patterns of anastomoses were categorized as three main, four subtype according to the following classification scheme proposed by Razavi et al. [12] (Fig. 1).
Type Ia was determined as that the ovarian artery was a major source of blood supply to the fibroids with anastomoses to intramural uterine artery, which the flow in the tubal artery was toward the uterus, without evidence of retrograde reflux in the direction of the ovary. Type Ib was determined as that the ovarian artery supplied the fibroids in a similar manner as that of Ia. But, flow toward the uterus in the tubal artery had reflux into the ovarian artery on the pre-embolization selective uterine angiogram. Type II was determined as that the ovarian artery supplied the fibroids directly and independent of the uterine artery. Type III was determined as that flow in the tubal artery was overall toward the ovary on selective uterine angiogram. All angiograms were evaluated by two experienced interventional radiologists and angiographic anastomotic types were determined by a consensus.
3. Pelvic MRI
Pelvic MRI was performed on GE Signa 1.5-T MRImager (GE Medical Systems, Milwaukee, WI, USA). The routine pelvic MRI protocol for evaluating women with suspected leiomyomas included axial and coronal T2-weighted images (repetition time/echo time [TR/TE] 3,500~4,000/100~120 msec; field of view [FOV] 30~34 cm; slice thickness 9~10 mm; pixel size 512×192), axial T1-weighted images (TR/TE 300~950; TE 14/92 msec; FOV 32~36 cm; slice thickness 10 mm, pixel size 288~320×160~224). Follow-up pelvic MRI consisted of axial and coronal T2-weighted images, axial T1-weighted images and axial, sagittal T1-weighted imaging after the administration of 0.1 mmol/kg dose of gadopentetate dimeglumine (Magnevist®, Berlex Pharmaceuticals, Wayne, NJ, USA). The parameters of the enhanced MRI were TR/TE 3.5~4.6/0.85~1.2 msec, FOV 30~40 cm, slice thickness 4 mm, pixel size 288~320×192.
Pelvic MRI was obtained at 1~6 months after the procedure to document the effects of UAE. Patients underwent another MRI after 6 months if they had unchanged or aggravated pre-procedural symptoms, new symptoms, and/or clinical concern for infection.
If MRI exhibits gadolinium enhancement on fibroid with presence of anastomoses between the ovarian and uterine arteries, volume changes of fibroid were documented by MR (magnetic resonance) images.
4. Evaluation of the effectiveness
It was reviewed that the pre-procedural and post-procedural clinical reports if the patient's symptoms and signs had been changed or aggravated. Post-procedural clinical follow-ups were performed in 12 months and annually thereafter. Clinical failure was defined as persistence or aggravation of symptoms at 1~6 months of follow-up and recurrence as return of symptoms [17]. Treatment failure was defined as peripheral residual enhancement or no volumetric decrease of fibroid on follow-up MRI in clinical failure women.
5. Statistical analysis
Chi-square test was used to compare mean treatment failure difference between patients with communication and without communication. Excel software (Microsoft, Redmond, WA, USA) was used for statistical analysis. Statistical significance was defined as a probability value below 0.05.
Results
Of the all 169 patients, three patients had an associated disease such as adenomyosis. They did not demonstrate the evidence of the focal enhancement on follow-up enhanced MRI. These three patients with adenomyosis were excluded, even though two of them complained of no symptomatic relief. Three patients demonstrated additional extrauterine collaterals such as inferior epigastric artery, lumbar artery, and umbilical artery. One of them underwent hysterectomy secondary to recurrence of menorrhagia due to an extrauterine blood supply. The other two patients had no symptomatic recurrence during the following period. They were excluded taking no account of the treatment failure of this study.
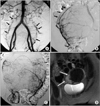
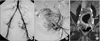
Fifty six patients had anastomoses between uterine artery and ovarian artery on pre-embolization angiography (56/163, 34.4%). Angiographic subtypes were type Ia in 19 patients, type Ib in 16 patients, type II in 11 patients and type III in 10 patients (Table 1). Of all patients, 10 patients showed the peripheral focal enhancements of the leiomyomas on follow-up enhanced MR images (10/163, 6.1%), one of them showed enlargement of the leiomyoma. Of the patients with type Ia anastomoses between uterine artery and ovarian artery on pre-embolization angiography, three patients showed the peripheral focal enhancement of the leiomyomas on follow-up enhanced MR imagings (3/19, 15.8%) (Fig. 2). One patient with type Ib (1/16, 6.3%) showed focal enhancements of the leiomyomas on follow-up enhanced MR images. Other 6 patients among the one who showed the peripheral focal enhancements of the leiomyomas on follow-up enhanced MR images had no anastomoses between uterine artery and other vessels (6/107, 5.7%) (Fig. 3).
Sixteen patients, who did not benefit from the procedure owing to persistence of symptoms, were considered as clinical failure (9.8%) (Table 1). Ten of these 16 patients (62.5%) showed persistent gadolinium enhancement of their dominant treated uterine leiomyomas on MR imaging. Four of them had communications between uterine artery and ovarian artery on pre-emboliation angiography with focal enhancement on follow-up enhancement MR images (Table 1, Fig. 2). Six of them had no communications between uteirine artery and ovarian artery without focal enhancement on follow-up enhanced MR images. The other patients showed complete tumor ischemia, as evidenced by lack of any gadolinium enhancement in the internal portion of the leiomyomas. These ten women were regarded as treatment failure. Of these treatment failure patients, three patients subsequently chose to undergo myomectomies, two patients received hysterectomy. Remaining five women did not make a consensus on additional uterine fibroid embolization or operation and decided just the clinical follow-up.
There was no statistical significance in treatment failure rate difference between patients with communication (4/56, 7.1%) and without communication (6/107, 5.7%) (P>0.05). But, treatment failure rate in patients with type Ia communication (15.8%) was higher than that without communication (5.7%, P<0.05).
Discussion
UAE is a minimally invasive alternative to traditional surgical options for managing symptomatic fibroids. Although UAE has a high success rate and a low complication rate, early treatment failure associated with temporary improvement or absence of clinical relief after UAE is reported in 6~20% [2,4,5,18-20]. Anastomoses between the ovarian and uterine arteries have been suggested as a cause of early treatment failure [9-12]. In the consecutive 163 symptomatic uterine fibroid cases, it was evaluated to demonstrate if the anastomoses between the ovarian and uterine arteries could be a cause of the treatment failure.
Early treatment failures are technical or anatomical origin, or sometimes associated with adenomyosis. Uterine fibroid with adenomyosis has been argued as a wrong indication for UAE because long-term results are not as good as in cases of only uterine fibroids [9,17,21,22] even though patients with adenomyosis reported clinical improvement in some papers [23-25]. Because of that reason, three uterine fibroid cases associated with adenomyosis were excluded.
The enhanced MR images were used for the determination treatment failure in this study. Even most articles have shown that incompletely infarcted leiomyomas after UAE could grow and result in recurrent symptoms by assessing interval changes in tumor and uterine volume [26], volume reduction could not be correlated with improvement in clinical symptom score [14]. Evaluation of the focal continuous enhancement of leiomyomas on MRI can be advocated to determine whether the treatment failure occurred or not [16].
Treatment failure rate difference showed no statistical significance according to the presence of angiographic communication between the ovarian and uterine arteries. But, the treatment failure rate (3/19, 15.8%) of the patients with type Ia anastomotic communication was higher than that of patients without communication (6/107, 5.7%). This anastomosis is a possible cause of the treatment failures, even though number of type Ia anastomoses was very small (Table 1). Current end point of the UAE has been accepted as a stasis of flow in the proximal uterine artery on the post-embolization angiographic imaging, and the operators need to stop the embolization procedure if intrauterine collaterals open as the resistance in the target vascular bed increases [11]. It can be inferred that patients with type Ia communication have the potential flow toward the uterus even after proximal uterine arteries were blocked by embolic materials.
Besides, shrinkage of existing leiomyomas does not prevent the uterus from developing new or resume to grow leiomyomas. It is because the difference of the vascular networks for leiomyomas (terminal artery) and normal myomas (large capillary network) accounts for the preferential target of the injected particles being leiomyoma [19]. Intramyoma vascularization disappears as early as 3 months after UAE but uterine vascularization is maintained as evidenced by contrast enhanced ultrasound [2,27-31].
And some reports had suggested that continued supply of the fibroids from the ovarian arteries via communication between ovarian artery and uterine artery should be considered as a possible mechanism in cases of the treatment failure following technically successful UAE [8,11,12].
This study has several important limitations. First, the sample size of women presenting treatment failure was relatively small. A large sample with obtained from multiple institutions would be helpful for definitive conclusion. Second, review of the MR images after primary UAE was not done in a blinded fashion, potentially introducing observer bias. Third, the same size and same type of embolic materials were not used. The uterine artery was embolized to complete stasis of flow in the main uterine artery by using either 355~500 µm polyvinyl alcohol particles or 500~700 µm triacryl gelatin microspheres.
The full clinical significance of collateral flow to fibroid has been emphasized [8,9,11,12,32,33] and it has been conceivable that many treatment failures from UAE are caused by unrecognized collateral fibroid feeders [8,9,11]. Although data presented here are too limited for conclusion, it can suggest the possibility that patients with type Ia anastomoses between the ovarian artery and the uterine artery could have the early treatment failure.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download