Abstract
Background
Circulating tumor cell (CTC) analysis is a promising new diagnostic field for estimating the risk for metastatic relapse and metastatic progression in patients with cancer.
Content
Different analytical systems for CTC isolation and detection have been developed as immunocytochemical and molecular assays, most including separation steps by size or biological characteristics, such as expression of epithelial- or cancer-specific markers. Recent technical advancements in CTC detection and characterization include methods based on multiplex reverse-transcription quantitative PCR and approaches based on imaging and microfilter and microchip devices. New areas of research are directed toward developing novel assays for CTC molecular characterization. QC is an important issue for CTC analysis, and standardization of micrometastatic cell detection and characterization methodologies is important for the incorporation of CTCs into prospective clinical trials to test their clinical utility. The molecular characterization of CTCs can provide important information on the molecular and biological nature of these cells, such as the status of hormone receptors and epidermal and other growth factor receptor family members, and indications of stem-cell characteristics. This information is important for the identification of therapeutic targets and resistance mechanisms in CTCs as well as for the stratification of patients and real-time monitoring of systemic therapies.
Summary
CTC analysis can be used as a liquid biopsy approach for prognostic and predictive purposes in breast and other cancers. In this review we focus on state-of-the-art technology platforms for CTC isolation, imaging, and detection; QC of CTC analysis; and ongoing challenges for the molecular characterization of CTCs.
Figures and Tables
Fig. 1
Main approaches for CTC isolation-enrichment. (A) Enrichment by density-gradient centrifugation in the presence of ficol. (B) Immunomagnetic separation [Fehm et al. (18), Königsberg et al. (19), Sieuwerts et al. (20), Mostert et al. (21), Schindlbeck et al. (22), Deng et al. (23)]. (B1) Negative selection through removal of leukocytes by anti-CD45; (B2) positive selection through an antibody against a pan-epithelial differentiation antigen, EpCAM; (B3) combined use of antibodies against CTC surface markers (anti-CD146, anti-CD176, anti-CK-19, and others). (C) ISET system [Vona et al. (24)]. (D) Microfluidic device: the CTC chip captures EpCAM-expressing cells in peripheral blood by use of anti-EpCAM-coated microposts [Nagrath et al. (27)]. (E) A portable filter-based microdevice filtration based on the size difference between CTCs and human blood cells [Lin et al. (25), Zheng et al. (26)].
Abbreviations: PBMCs, peripheral blood mononuclear cells; PDMS, polydimethylsiloxane.

Fig. 2
Main approaches for CTC detection and molecular characterization. (A) Image-based approaches: (A1) classic ICC; (A2) CellSearch system (FDA cleared); (A3) Ariol system; (A4) laser-scanning cytometry; (A5) EPISPOT assay (detects tumor-specific proteins released by CTCs). (B) Molecular assays, based on nucleic acid analysis in CTCs: (B1) classic RT-PCR; (B2) multiplex RT-PCR, AdnaTest BreastCancer; (B3) RT-qPCR; (B4) liquid bead array.
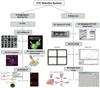
Table 1
Overview of analytical methodologies for the detection and molecular characterization of CTCs

Abbreviations: hMAM, human MAM; CEA, carcinoembryonic antigen; GABA, γ-aminobutyric acid; PTPRC, protein tyrosine phosphatase, receptor type, C; CCNE2, cyclin E2; EMP2, epithelial membrane protein 2; MAL2, myelin and lymphocyte 2; PPIC, peptidylprolyl isomerase C; SLC6A8, solute carrier family 6 (neurotransmitter transporter, creatine), member 8; BST, bone marrow stromal cell antigen; MAGE, melanoma-associated antigen; PBGD, porphobilinogen deaminase.
References
1. Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Australia. 1869. 14:146–147.
2. Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003. 3:453–458.

4. Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009. 6:339–351.

5. Hayes DF, Smerage JR. Is there a role for circulating tumor cells in the management of breast cancer? Clin Cancer Res. 2008. 14:3646–3650.

6. Kasimir-Bauer S. Circulating tumor cells as markers for cancer risk assessment and treatment monitoring. Mol Diagn Ther. 2009. 13:209–215.

7. Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005. 353:793–802.

8. Stathopoulou A, Mavroudis D, Perraki M, Apostolaki S, Vlachonikolis I, Lianidou E, et al. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002. 20:3404–3412.

9. Xenidis N, Vlachonikolis I, Mavroudis D, Perraki M, Stathopoulou A, Lianidou E, et al. Peripheral blood circulating cytokeratin-19 mRNA-positive cells after the completion of adjuvant chemotherapy in patients with operable breast cancer. Ann Oncol. 2003. 14:849–855.

10. Xenidis N, Perraki M, Kafousi M, Apostolaki S, Lianidou ES, Georgoulias V, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in nodenegative breast cancer patients. J Clin Oncol. 2006. 24:3756–3762.

11. Ignatiadis M, Kallergi G, Ntoulia M, Lianidou E, Georgoulias V, Mavroudis D, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res. 2008. 14:2593–2600.

12. Ignatiadis M, Xenidis N, Perraki M, Lianidou E, Sotiriou C, Georgoulias V, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007. 25:5194–5202.

13. Xenidis N, Ignatiadis M, Apostolaki S, Perraki M, Georgoulias V, Mavroudis D, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009. 27:2177–2184.

14. Cristofanilli M, Budd GT, Ellis MJ, Allard WJ, Terstappen LW, Hayes DF, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004. 351:781–791.

15. Mostert B, Sleijfer S, Foekens JA, Gratama JW. Circulating tumor cells (CTCs): detection methodsand their clinical relevance in breast cancer. Cancer Treat Rev. 2009. 35:463–474.

16. Lianidou ES, Mavroudis D, Sotiropoulou G, Agelaki S, Pantel K. What's new on circulating tumor cells? A meeting report. Breast Cancer Res. 2010. 12:307.

17. Tibbe AG, Miller MC, Terstappen LW. Statistical considerations for enumeration of circulating tumor cells. Cytometry A. 2007. 71:154–162.

18. Fehm T, Solomayer EF, Meng S, Tucker T, Lane N, Wang J, et al. Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy. 2005. 7:171–185.

19. Königsberg R, Obermayr E, Bises G, Pfeiler G, Gneist M, Wrba F, et al. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 2011. 50:700–710.

20. Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009. 101:61–66.

21. Mostert B, Kraan J, Bolt-de Vries J, van der Spoel P, Sieuwerts AM, Schutte M, et al. Detection of circulating tumor cells in breast cancer may improve through enrichment with anti-CD146. Breast Cancer Res Treat. 2011. 127:33–41.

22. Schindlbeck C, Stellwagen J, Jeschke U, Karsten U, Rack B, Janni W, et al. Immunomagnetic enrichment of disseminated tumor cells in bone marrow and blood of breast cancer patients by the Thomsen-Friedenreich-Antigen. Clin Exp Metastasis. 2008. 25:233–240.

23. Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res. 2008. 10:R69.

24. Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000. 156:57–63.
25. Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, Scher HI, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. 2010. 16:5011–5018.

26. Zheng S, Lin HK, Lu B, Williams A, Datar R, Cote RJ, et al. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices. 2011. 13:203–213.

27. Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007. 12. 20. 450:1235–1239.

28. Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A. 2010. 107:18392–18397.

29. Adams AA, Okagbare PI, Feng J, Hupert ML, Patterson D, Göttert J, et al. Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. J Am Chem Soc. 2008. 130:8633–8641.

30. Wang X, Qian X, Beitler JJ, Chen ZG, Khuri FR, Lewis MM, et al. Detection of circulating tumor cells in human peripheral blood using surfaceenhanced raman scattering nanoparticles. Cancer Res. 2011. 71:1526–1532.

31. Pachmann K, Clement JH, Schneider CP, Willen B, Camara O, Pachmann U, et al. Standardized quantification of circulating peripheral tumor cells from lung and breast cancer. Clin Chem Lab Med. 2005. 43:617–627.

32. Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007. 13:920–928.

33. Balic M, Rapp N, Stanzer S, Lin H, Strutz J, Szkandera J, et al. Novel immunofluorescence protocol for multimarker assessment of putative disseminating breast cancer stem cells. Appl Immunohistochem Mol Morphol. 2011. 19:33–40.

34. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009. 55:611–622.

35. Slade MJ, Smith BM, Sinnett HD, Cross NC, Coombes RC. Quantitative polymerase chain reaction for the detection of micrometastases in patients with breast cancer. J Clin Oncol. 1999. 17:870–879.

36. Stathopoulou A, Gizi A, Perraki M, Apostolaki S, Malamos N, Mavroudis D, et al. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res. 2003. 9:5145–5151.
37. Stathopoulou A, Ntoulia M, Perraki M, Apostolaki S, Mavroudis D, Malamos N, et al. A highly specific real-time RT-PCR method for the quantitative determination of CK-19 mRNA positive cells in peripheral blood of patients with operable breast cancer. Int J Cancer. 2006. 119:1654–1659.

38. Ring AE, Zabaglo L, Ormerod MG, Smith IE, Dowsett M. Detection of circulating epithelial cells in the blood of patients with breast cancer. Comparison of three techniques. Br J Cancer. 2005. 92:906–912.

39. Fehm T, Braun S, Muller V, Janni W, Naume B, Pantel K, et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006. 107:885–892.

40. Obermayr E, Sanchez-Cabo F, Tea MK, Singer CF, Krainer M, Fischer MB, et al. Assessment of a six gene panel for the molecular detection of circulating tumor cells in the blood of female cancer patients. BMC Cancer. 2010. 10:666.

41. Reinholz MM, Nibbe A, Jonart LM, Houghton R, Zehentner B, Roche PC, et al. Evaluation of a panel of tumor markers for molecular detection of circulating cancer cells in women with suspected breast cancer. Clin Cancer Res. 2005. 11:3722–3732.

42. Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelialmesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009. 11:R46.

43. Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D, et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RTPCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009. 11:R59.

44. Sieuwerts AM, Kraan J, Bolt-de Vries J, van der Spoel P, Mostert B, Martens JW, et al. Molecular characterization of circulating tumor cells in large quantities of contaminating leukocytes by a multiplex real-time PCR. Breast Cancer Res Treat. 2009. 118:455–468.

45. Markou A, Strati A, Malamos N, Georgoulias V, Lianidou ES. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin Chem. 2011. 57:421–430.

46. Alix-Panabières C, Vendrell JP, Slijper M, Pellé O, Barbotte E, Mercier G, et al. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res. 2009. 11:R39.

47. Khleif SN, Doroshow JH, Hait WN. AACR-FDA-NCI Cancer Biomarkers Collaborative. AACR-FDA-NCI Cancer Biomarkers Collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin Cancer Res. 2010. 16:3299–3318.

48. Fehm T, Müller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010. 124:403–412.

49. Kraan J, Sleijfer S, Strijbos MH, Ignatiadis M, Peeters D, Pierga JY, et al. External quality assurance of circulating tumor cell enumeration using the CellSearch® system: a feasibility study. Cytometry B Clin Cytom. 2011. 80:112–118.

50. Balic M, Dandachi N, Hofmann G, Samonigg H, Loibner H, Obwaller A, et al. Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytometry B Clin Cytom. 2005. 68:25–30.

51. Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh RF, et al. Molecular biomarker analyses using circulating tumor cells. PLoS One. 2010. 5:e12517.

52. Becker S, Becker-Pergola G, Banys M, Krawczyk N, Wallwiener D, Solomayer E, et al. Evaluation of a RT-PCR based routine screening tool for the detection of disseminated epithelial cells in the bone marrow of breast cancer patients. Breast Cancer Res Treat. 2009. 117:227–233.

53. Van der Auwera I, Peeters D, Benoy IH, Elst HJ, Van Laere SJ, Prové A, et al. Circulating tumour cell detection: a direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br J Cancer. 2010. 102:276–284.

54. Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancerclinical applications. Nat Rev Clin Oncol. 2010. 7:693–701.

55. Kallergi G, Markomanolaki H, Giannoukaraki V, Papadaki MA, Strati A, Lianidou ES, et al. Hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2009. 11:R84.

56. Tewes M, Aktas B, Welt A, Mueller S, Hauch S, Kimmig R, et al. Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treat. 2009. 115:581–590.

57. Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Uhr J, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A. 2004. 101:9393–9398.
58. Bozionellou V, Mavroudis D, Perraki M, Stathopoulou A, Lianidou E, Georgoulias V, et al. Trastuzumab administration can effectively target chemotherapy-resistant cytokeratin-19 messenger RNA-positive tumor cells in the peripheral blood and bone marrow of patients with breast cancer. Clin Cancer Res. 2004. 10:8185–8194.

59. Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, Komor M, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010. 16:2634–2645.

60. Cao S, Li Y, Li J, Li CF, Zhang W, Yang ZQ, et al. Quantitative determination of HER2 expression by confocal microscopy assay in CTCs of breast cancer. Oncol Rep. 2010. 23:423–428.

61. Ignatiadis M, Rothé F, Chaboteaux C, Durbecq V, Rouas G, Criscitiello C, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011. 6:e15624.

62. Apostolaki S, Perraki M, Pallis A, Bozionelou V, Agelaki S, Kanellou P, et al. Circulating HER2 mRNA-positive cells in the peripheral blood of patients with stage I and II breast cancer after the administration of adjuvant chemotherapy: evaluation of their clinical relevance. Ann Oncol. 2007. 18:851–858.

63. Flores LM, Kindelberger DW, Ligon AH, Capelletti M, Fiorentino M, Loda M, et al. Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br J Cancer. 2010. 102:1495–1502.

64. Munzone E, Nolé F, Goldhirsch A, Botteri E, Esposito A, Zorzino L, et al. Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer. 2010. 10:392–397.

65. Payne RE, Yagüe E, Slade MJ, Apostolopoulos C, Jiao LR, Ward B, et al. Measurements of EGFR expression on circulating tumor cells are reproducible over time in metastatic breast cancer patients. Pharmacogenomics. 2009. 10:51–57.

66. Liu Z, Fusi A, Schmittel A, Tinhofer I, Schneider A, Keilholz U. Eradication of EGFR-positive circulating tumor cells and objective tumor response with lapatinib and capecitabine. Cancer Biol Ther. 2010. 10:860–864.

67. Ntoulia M, Stathopoulou A, Ignatiadis M, Malamos N, Mavroudis D, Georgoulias V, et al. Detection of Mammaglobin A-mRNA-positive circulating tumor cells in peripheral blood of patients with operable breast cancer with nested RT-PCR. Clin Biochem. 2006. 39:879–887.

68. Diehn M, Cho RW, Clarke MF. Therapeutic implications of the cancer stem cell hypothesis. Semin Radiat Oncol. 2009. 19:78–86.

69. Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004. 10:8152–8162.

70. Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008. 27:6120–6130.

71. Bonnomet A, Brysse A, Tachsidis A, Waltham M, Thompson EW, Polette M, et al. Epithelial-tomesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia. 2010. 15:261–273.

72. Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006. 12:5615–5621.

73. Theodoropoulos PA, Polioudaki H, Agelaki S, Kallergi G, Saridaki Z, Mavroudis D, et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010. 288:99–106.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download