Abstract
Background
The effect of pravastatin on insulin resistance (IR) is controversial and poorly studied in prediabetes.
Methods
This study was performed in hyperglycemic patients at Saint Carollo Hospital from January 1, 2013 to December 31, 2015. Among them, we selected 40 patients (24 prediabetes and 16 new onset diabetes [NOD]) who had been treated with pravastatin 20 mg daily for 2 or 4 months and in whom fasting insulin and fasting glucose had been measured before and after administration of pravastatin. IR was defined as a fasting insulin level ≥ 12.94 μU/mL, homeostasis model for IR (HOMA-IR) ≥ 3.04 or quantitative insulin sensitivity check index (QUICKI) ≤ 0.32.
Results
Pravastatin treatment decreased total cholesterol and low-density lipoprotein cholesterol levels by 25.2% and 32.3% respectively (P = 0.000 for all), but did not affect fasting insulin level, HOMA-IR, or QUICKI in total, prediabetes, and NOD groups. Prevalence of IR was significantly different between prediabetes and NOD groups both before and after pravastatin treatment (0% versus 37.5%, P = 0.001), but pravastatin treatment did not affect the prevalence of IR in the prediabetes or NOD group. Fasting glucose level was not significantly different before and after pravastatin treatment in prediabetes (106.8 ± 6.4 mg/dL versus 103.8 ± 8.4 mg/dL, P = 0.223) but was significantly different in the NOD group (171.5 ± 70.1 mg/dL versus 124.4 ± 26.7 mg/dL,P = 0.017).
References
1. Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Study Group. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. 2002; 359:1379–87.
2. Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kieldsen SE, Kristinsson A, Mclnnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicenter randomized controlled trial. Lancet. 2003; 361:1149–58.
3. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic hypertensive patients randomized to pravastatin vs usual care. JAMA. 2002; 288:2998–3007.
4. Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002; 360:1623–30.

5. Paolisso G, Barbagallo M, Petrella G, Ragno E, Barbieri M, Giordano M, Varricchio M. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients. Atherosclerosis. 2000; 150:121–7.

6. Chan P, Tomlinson B, Lee CB, Pan WH, Lee YS. Beneficial effects of pravastatin on fasting hyperinsulinemia in elderly hypertensive hypercholesterolemic subjects. Hypertension. 1996; 28:647–51.

7. Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane P, Cobbe SM. Long-Term Follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007; 357:1477–86.

8. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative metaanalysis of randomised statin trials. Lancet. 2010; 375:735–42.

9. Baker WL, Talati R, White CM, Coleman CI. Differing effect of statins on insulin sensitivity in non-diabetics: a systematic review and metaanalysis. Diabetes Res Clin Pract. 2010; 87:98–107.

10. Ma T, Tien L, Fang CL, Liou YS, Jong GP. Statins and new-onset diabetes: a retrospective longitudinal cohort study. Clin Ther. 2012; 34:1977–83.

11. Park SM, Lim MK, Jung KW, Shin SA, Yoo KY, Yun YH, Huh BY. Prediagnosis smoking, obesity, insulin resistance, and second primary cancer risk in male cancer survivors: National Health Insurance Corporation Study. J Clin Oncol. 2007; 25:4835–43.
12. Lee S, Choi S, Kim HJ, Chung YS, Lee KW, Lee HC, Huh KB, Kim DJ. Cutoff values of surrogate measures of insulin resistance for metabolic syndrome in Korean nondiabetic adults. J Korean Med Sci. 2006; 21:695–700.

13. American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013; 36(Suppl 1):S11–66.
14. Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010; 55:1209–16.

15. Cho Y, Choe E, Lee YH, Seo JW, Choi Y, Yun Y, Wang HJ, Ahn CW, Cha BS, Lee HC, Kang ES. Risk of diabetes in patients treated with HMG-CoA reductase inhibitors. Metabolism. 2015; 64:482–8.

16. Vallejo-Vaz AJ, Kondapally Seshasai SR, Kurogi K, Michishita I, Nozue T, Sugiyama S, Tsimikas S, Yoshida H, Ray KK. Effect of pitavastatin on glucose, HbA1c and incident diabetes: a metaanalysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis. 2015; 241:409–18.

17. Gannagé-Yared MH, Azar RR, Amm-Azar M, Khalifé S, Germanos-Haddad M, Neemtallah R, Halaby G. Pravastatin does not affect insulin sensitivity and adipocytokines levels in healthy nondiabetic patients. Metabolism. 2005; 54:947–51.

18. Anagnostis P, Selalmatzidou D, Polyzos SA, Panagiotou A, Slavakis A, Panagiotidou A, Athyros VG, Karagiannis A, Mikhailidis DP, Kita M. Comparative effects of rosuvastatin and atorvastatin on glucose metabolism and adipokine levels in non-diabetic patients with dyslipidaemia: a prospective randomized open-label study. Int J Clin Pract. 2011; 65:679–83.
19. Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulindependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993; 329:1988–92.

20. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002; 51:2796–803.
21. Robinson JG. Statins and diabetes risk: how real is it and what are the mechanisms? Curr Opin Lipidol. 2015; 26:228–35.
Fig. 1.
Fasting insulin level, homeostasis model assessment of insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) before and after pravastatin treatment. Black, new onset diabetes mellitus; Gray, prediabetes.
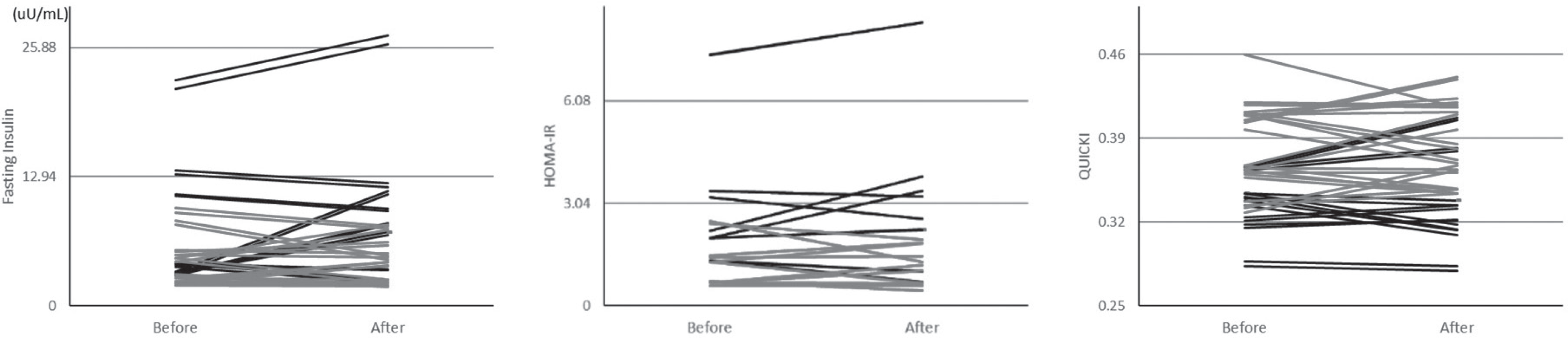
Table 1.
Baseline characteristics
Table 2.
The comparison of lipid profile, HOMA-IR and QUICKI before and after pravastatin treatment
| Variables | Total | Prediabetes | New onset diabetes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P-value | Before | After | P-value | Before | After | P-value | |
| Insulin (μU/mL) | 6.03 ± 5.03 | 6.81 ± 5.72 | 0.575 | 4.41 ± 2.38 | 4.31 ± 2.10b | 0.57 | 8.07 ± 6.71 | 10.16 ± 7.23b | 0.098 |
| C-peptide (ng/mL) | 1.68 ± 0.89 | 1.96 ± 1.29 | 0.247 | 1.34 ± 0.53a | 1.35 ± 0.64b | 0.937 | 2.2 ± 1.1a | 2.9 ± 1.5b | 0.093 |
| Total cholesterol (mg/dL) | 229.6 ± 37.8 | 171.8 ± 25.5 | 0.000 | 228.1 ± 33.7 | 174.4 ± 23.5 | 0.002 | 231.8 ± 45.6 | 167.8 ± 29.4 | 0.025 |
| LDL cholesterol (mg/dL) | 148.7 ± 22.7 | 100.7 ± 21.9 | 0.000 | 150.3 ± 22.1 | 102.5 ± 18.4 | 0.002 | 146.3 ± 24.9 | 98.0 ± 27.4 | 0.017 |
| HDL cholesterol (mg/dL) | 59.9 ± 13.1 | 56.7 ± 12.0 | 0.243 | 58.9 ± 15.3 | 58.1 ± 14.0 | 0.695 | 61.4 ± 9.4 | 54.5 ± 8.5 | 0.176 |
| Triglycerides (mg/dL) | 127.7 ± 63.3 | 108.2 ± 62.4 | 0.040 | 111.2 ± 55.0a | 102.3 ± 69.2 | 0.131 | 152.4 ± 70.4a | 117.0 ± 53.7 | 0.093 |
| Fasting glucose (mg/dL) | 132.7 ± 53.8 | 112.0 ± 20.3 | 0.007 | 106.8 ± 6.4a | 103.8 ± 8.4b | 0.223 | 171.5 ± 70.1a | 124.4 ± 26.7b | 0.017 |
| HOMA-IR | 1.88 ± 1.54 | 1.96 ± 1.80 | 0.881 | 1.21 ± 0.67a | 1.14 ± 0.54b | 0.647 | 2.88 ± 1.93a | 3.19 ± 2.31b | 0.301 |
| QUICKI | 0.364 ± 0.04 | 0.36 ± 0.04 | 0.732 | 0.38 ± 0.03a | 0.38 ± 0.03b | 0.927 | 0.33 ± 0.03a | 0.34 ± 0.04b | 0.836 |
Values are presented as mean ± standard deviation. HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; LDL, low-density lipoprotein; HDL, high-density lipoprotein.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download