Abstract
PURPOSE
Silk fibroin (SF) is a new degradable barrier membrane for guided bone regeneration (GBR) that can reduce the risk of pathogen transmission and the high costs associated with the use of collagen membranes. This study compared the efficacy of SF membranes on GBR with collagen membranes (Bio-Gide®) using a rat calvarial defect model.
MATERIALS AND METHODS
Thirty-six male Sprague Dawley rats with two 5 mm-sized circular defects in the calvarial bone were prepared (n=72). The study groups were divided into a control group (no membrane) and two experimental groups (SF membrane and Bio-Gide®). Each group of 24 samples was subdivided at 2, 4, and 8 weeks after implantation. New bone formation was evaluated using microcomputerized tomography and histological examination.
RESULTS
Bone regeneration was observed in the SF and Bio-Gide®-treated groups to a greater extent than in the control group (mean volume of new bone was 5.49 ± 1.48 mm3 at 8 weeks). There were different patterns of bone regeneration between the SF membrane and the Bio-Gide® samples. However, the absolute volume of new bone in the SF membrane-treated group was not significantly different from that in the collagen membrane-treated group at 8 weeks (8.75 ± 0.80 vs. 8.47 ± 0.75 mm3, respectively, P=.592).
CONCLUSION
SF membranes successfully enhanced comparable volumes of bone regeneration in calvarial bone defects compared with collagen membranes. Considering the lower cost and lesser risk of infectious transmission from animal tissue, SF membranes are a viable alternative to collagen membranes for GBR.
Guided bone regeneration (GBR) is a widely used technique for the replacement of insufficient bone during an implant procedure. The main role of the barrier membrane in GBR is to exclude epithelial or fibroblast infiltration and to promote bone regeneration by providing growth factors in the osteogenesis cavity.1,2,3,4,5 A number of suitable nondegradable and degradable membranes have been developed.6 In a recent clinical study, there was no statistically significant difference observed in the survival rate of implants over a 10-year period between groups treated with GBR and groups treated with pristine bone.7 In addition, there was no marked clinical or radiologic difference between groups treated with nondegradable and degradable membranes. This study demonstrates that GBR is a safe and effective treatment option, and that the choice of the type of membrane does not affect the long-term clinical outcomes.
Nevertheless, the use of an appropriate membrane in GBR during implant therapy is important in terms of operator convenience, and for the safety and comfort of the patient. Nondegradable membranes, such as titanium mesh or polytetrafluoroethylene, may be ideal barrier membranes for GBR because of their excellent biocompatibility and clinical manageability.8,9 However, nondegradable membranes have a critical disadvantage in that they require the removal of the membrane, which involves a high risk of infection.10,11 The use of degradable membranes overcomes this disadvantage, and many degradable biomaterials, such as collagen, polyglycolide, and polylactic acid, have been identified.12,13,14 Synthetic degradable membranes made from polyglycolide or polylactic acid may be unsuitable because of possible adverse events, such as extensive host immune reaction, postoperative swelling, and acid production, which could resolve nearby bone.15,16,17 The majority of clinically used degradable membranes are made of bovine collagen material, which is now a widely used commercial material.18 However, there are potential shortcomings in the use of collagen membranes. Considering that collagen membranes are derived from parts of animals, the transmissibility of infectious pathogens between the veterinary medicinal products and humans is of concern in GBR procedures using collagen membranes.19,20 A consensus of using safe materials that can be used as an alternative for animalderived materials, such as collagen, has arisen, and trials into developing replacement materials, such as biodegradable polymers or other synthetic degradable materials, should be considered in tissue regeneration therapy.21
Some studies have attempted to develop novel degradable membranes that are both safe and suitable for GBR. Among these, silk fibroin was suggested as a good candidate material for GBR. There is a long history of the use of silk spun into fibers by the silkworm in various clinical settings.22 Silk fibers are made from sericin, which is an antigenic gum-like protein, and fibroin, which is the core filament responsible for elasticity of silk.22,23 Native silk can induce adverse allergic reactions when implanted into the human body, and sericin has been identified as the major antigenic protein.22 Recently, silk fibroin, which is free from sericin, has been developed, and it has many attractive properties for use as a scaffold in tissue regeneration therapy.24 Decorated silk films have shown their suitability for bone regeneration therapy by demonstrating a sufficient osteoblast response, and calcium deposition and nodule formation in vitro.25 Kim et al.26 performed an animal study to evaluate the use of silk fibroin for new bone regeneration in vivo. Quantitative new bone growth related to ALPase activity and mineralization were monitored in calvarium-defected animal models, and favorable data were shown compared with control groups. This study strongly suggested that silk fibroin membranes are useful as a barrier material for GBR.
We hypothesized that silk membranes would show a greater efficacy for bone regeneration than nontreated groups, and similar or better efficacy than the widely used collagen membranes. A comparable efficacy for bony regeneration compared with collagen membranes would support the use of alternative silk fibroin membranes. However, there are no known data for comparing bone regeneration between silk fibroin membranes and collagen membranes in GBR.
The aim of this study was to compare the efficacy of silk fibroin membranes with that of the widely used collagen membrane, Bio-Gide®, for bone regeneration using surgically prepared calvarial defects in rats. The amount and histological changes of bone regeneration using silk fibroin membranes and collagen membranes were compared using microcomputerized tomography (micro-CT) and histological evaluation.
The silk fibroin and collagen membranes used were prepared as follows. First, silk fibroin membranes were made from native silk. Silkworm cocoons were harvested by the Rural Development Administration (Suwon, Republic of Korea), and then raw silk fibers were prepared. The raw silk fibers were degummed twice using a 0.5% sodium carbonate solution, and then washed with distilled water. The degummed fibers were dissolved in a solution containing CaCl2, ethanol, and H2O (mixed in a molar ratio of 1 : 2 : 8). After being subjected to dialysis for 4 days to remove the CaCl2 and ethanol, the resulting silk fibroin solution was stored in a refrigerator. The silk fibroin solution was poured in a polystyrene petri dish (Cat. #10,091, ID = 90 mm, SPL Life Sciences Co Ltd, Pocheon, Republic of Korea), and a transparent silk fibroin membrane was obtained. The collagen-type barrier membranes, Bio-Gide®, were purchased from Geistlich Pharma AG, Wolhusen, Switzerland.
The study groups were divided into three groups: a control group (no membrane) and two experimental groups (silk fibroin and Bio-Gide® membranes). Each group of 24 samples was subdivided into three subgroups of eight samples for a time course analysis (at 2, 4, and 8 weeks after implantation). Two circular calvarial bony defects were prepared in 36 male Sprague Dawley rats, providing 72 samples in total. Before the surgical procedure was carried out, the animals were anesthetized using an intramuscular injection of 0.1 cm3/100 g tiletamine and zolazepam (Zoletil, Bayer Korea, Seoul, Korea) and 0.04 cm3/100 g xylazine hydrochloride (Rompun, Bayer Korea, Seoul, Korea). Once fully anaesthetized, the animals were injected preoperatively with 2% lidocaine and epinephrine at the surgery site. A 2 cmsized longitudinal skin incision was made in the scalp along the sagittal suture line, and the musculature and the periosteum were exposed under the skin to allow for the periosteal dissection procedure. Two symmetrical round bone defects, 5 mm in diameter and full thickness, were made in the dorsal part of the left and right parietal bones using a dental trephine bur under sterile saline irrigation (Fig. 1). 27 During the creation of the bone defects, great care was taken to avoid involvement of the midsagittal suture in the bone defect, and any damage to the dura mater or the superior sagittal sinus. For the silk fibroin membrane-treated group (n=24) and the Bio-Gide®-treated group (n=24), the assigned sheet of membrane (silk fibroin or Bio-Gide®) was implanted so as to cover the bone defects fully (Fig. 1). The membranes extended about 4 mm beyond the defect margins and were fixed with resorbable sutures at the four edges of the outer membrane. The bone defects were left uncovered in the control group (n=24). After the surgical procedure on the calvarial bone, the periosteum and skin wound were sutured using absorbable and 3-0 silk sutures, respectively. In line with the guidelines of the Danish Animal Research Council, all the animals were housed in plastic cages at a temperature of 22℃ with 12 hour lightdark cycles. The animals had free access to tap water and were fed a standard laboratory diet. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Bioventure Incubation Center, Hanbat National University, Daejeon, Korea.
Within each group (n=24), three subgroups of animals were euthanized at 2, 4, and 8 weeks after surgery and bone formation was analyzed. After dissection of the scalp and periosteal tissue, the defected bone sites were exposed and removed along with all surrounding bone and soft tissues. Calvarial bone specimens measuring 25 × 12 × 3 mm3 (length × width × height) were separated from the defected bone sites and fixed in 10% formalin for radiologic bone mass analysis. Extracted calvarial specimens were scanned at a spatial resolution of 35 µm3 using micro-CT (Skyscan 1076, Skyscan NV, Belgium). Scanning parameters were set at voltage = 100 kV, current = 100 µA, exposure time = 474 ms, aluminum filter = 0.5, and rotation step = 0.5°. Bone volumes were easured using a CT-AN 1.8 analyzer (Skyscan NV, Belgium) to evaluate new bone formation. The values around the region of interest were analyzed over a diameter of 5 mm in the analysis of newly formed bone.
The calvarial specimens taken for histological examination were dehydrated in ethanol, and then decalcified by submersion in 5% nitric acid for a period of 1 week. Specimens were divided into right and left portions relative to the midline sagittal suture. The two separated portions were embedded in paraffin blocks, cut into 5 µm slices, and stained with hematoxylin and eosin. Sliced tissue sections were collected from the middle area of the defect sites and were examined using optical microscopy.
The sample size was calculated according to the difference in new bone formation (mm3) between groups treated with a guided membrane and the nontreated control group. Based on a previous similar in vivo study,28 we predicted that there would be a difference of approximately 6 mm3 in new bone formation between the treated and the nontreated groups, and the standard deviation was assumed to be 1 mm3. With an a level of 0.05 (two-tailed) and a power of 95%, an animal sample size of six animals for each group was determined to be sufficient for statistical testing of the difference between the groups. Considering an anticipated dropout of one to two samples during the study, we assigned eight samples to each experimental group. The independent variables were the three groups (control, silk fibroin membrane, and Bio-Gide® membrane) and the time after surgery (2, 4, and 8 weeks). The dependent variable was the volume of new bone. Statistical analysis of the newly formed bone volumes between the groups over time was performed using ANOVA tests on a parametric distribution. We analyzed data using the SPSS v 17.0 statistical software package (SPSS Inc., Chicago, IL, USA). Two-tailed P values less than 0.05 were considered statistically significant.
Out of the 36 animals that underwent surgical procedure, three rats died. Thirty-three rats survived the interventional surgery and healed well without significant weight reduction or postoperative infection. From each subgroup (total of 12 subgroups with 6-8 animals), the absolute volume (mm3) of new bone in the calvarial defects was analyzed using micro-CT. Histological assessment was performed using specimens from healed calvarial bone defects after the micro-CT examination had been carried out.
New bone formation and other related adherent tissue reactions at the bone margin were examined in each sample. At 2 weeks after surgery, there was no considerable difference in new bone formation between the control groups and both membrane-treated groups. In the control group, there was reduced bone regeneration at 4 weeks, and no significant increase in new bone formation was observed in the microscopic examination. By 8 weeks, infiltrating growths of fibrotic and epithelial tissue were observed in the defects (Fig. 2). In the collagen membrane-treated defects, a considerable volume of new bone from the bony margins of the defect was observed at 4 and 8 weeks. The silk fibroin membrane-treated defects had sufficient bone growth at 4 weeks, but more ingrowth of new bone seemed to occur by 8 weeks (Fig. 2). At 2 and 4 weeks after surgery, the silk fibroin and Bio-Gide® membranes were visible in the histological examination, but no remnant of either membrane was found under microscopic examination at 8 weeks (Fig. 2). No adverse tissue reactions were observed in any specimens.
The absolute volume of new bone formation as measured using micro-CT was significantly different between the control group, the Bio-Gide® group, and the silk fibroin group at 2, 4, and 8 weeks (P<.05) (Fig. 3, Table 1). Although the mean volume of bone formation increased in the control group, this was considerably lower than in the two membrane-treated groups over the 8-week period in the study. At 2 weeks, the mean volume of new bone formation was lower in the Bio-Gide® group compared with the other groups. However, by 4 weeks, the Bio-Gide® group had the largest volume of new bone formation of all the groups. At 8 weeks, the mean volume of new bone formation in the silk fibroin group increased significantly, and there was no significant difference from the mean volume of new bone formation in the Bio-Gide® group (8.75 ± 0.80 vs. 8.47 ± 0.75, respectively; P=.592).
Fig. 4 shows the growth of new bone as a function of time. This clearly shows there were different growth patterns among the three groups. In the control group, bone growth remained stable after 4 weeks with a relatively lower bone regeneration rate. The Bio-Gide® membrane-treated group underwent significant bone regeneration during 2-4 weeks and minimal growth thereafter. In contrast, in the silk fibroin membrane-treated group, there was consistent growth during the 2-8 week period.
In this study, the efficacy of silk fibroin membranes on new bone regeneration of calvarial defects in a rat model for GBR treatment was evaluated. This was compared with a control group and with a group receiving treatment with the widely used collagen membrane, Bio-Gide®. At 2, 4, and 8 weeks after treatment, we performed histological evaluation and assessed the amount of new bone formed using micro-CT. After treatment, both the Bio-Gide® and silk fibroin membranes showed high volumes of new bone formation in the calvarial defects compared with the control group. At 2 and 4 weeks, the volume and pattern of bone growth into the defects were different between the two membrane-treated groups. However, by 8 weeks, a similar level of bone regeneration was observed in the calvarial defects treated with each membrane compared with the calvarial defects in the nontreated group.
Micro-CT was performed instead of histomorphometrical analysis to compare new bone formation between the collagen- and SF membrane-treated groups, as the former is considered the gold standard. Traditionally, micro-CT has been used for quantifying three-dimensional trabecular bony structures.29 However, this technique can be used for measuring new bone generation by using it to analyze mineralization in zones of interest.30,31 Although, this simple technique cannot fully replace the conventional histomorphometric measurement, micro-CT has a number of advantages. This method does not scarify the study subjects, and it requires no special preparation during sectioning and staining, meaning that it is less time-consuming. Second, micro-CT can provide valid quantitative measurements of new bone formation, which supports three-dimensional reconstructions and volumetric measurements.31 The use of micro-CT as a tool for quantitative measurements of new bone generation has been validated in previous comparative studies.21,28,31,32
In this study, micro-CT was useful for determining the progression pattern of new bone formation over the 2-8-week period for each treatment group. In the control group (i.e., no membrane), some increase in the volume of new bone was observed after 2 and 4 weeks, but there was no significant growth from 4 to 8 weeks. Infiltration of epithelial and fibrotic cells into the bone defects may have occurred with early bone regeneration, which may have contributed to the lack of new bone generated, as they would have occupied the defect site from week 4 onward. This assumption was verified via histological examination of the control group after 8 weeks in our study.
In the collagen membrane-treated groups, a lower volume of new bone formation was observed at 2 weeks (mean new bone volume = 1.73 mm3) compared with the control group (mean new bone volume = 2.6 mm3). This observation can be explained by the results from the study of Gielkens et al.,28 which followed a similar study design and conditions to ours. In this study, micro-CT showed that higher bone regeneration occurred in the control group compared with the collagen membrane-treated group after 2 weeks. However, microradiography showed a higher percentage of defect closure in the collagen-treated group (60.2%) compared with the control group (22.8%).28 Micro-CT was used to calculate the absolute bone mass in three dimensions quantitatively. However, microradiography evaluated the rate of defect covering only two dimensions.28,33 In general, new bone generation at a defect site begins with defect closure. First, a thin layer of new bone forms; second, bone thickening occurs, which is followed by sufficient defect closure.12 In the collagen group, bone formation simultaneously began over a wide surface area of the defect at 2 weeks. However, bone thickening was slower, which resulted in a higher percentage of defect closure (60.2%) but the formation of a relatively lower volume of new bone. In contrast, the control group had a relatively lower percentage of defect closure (22.8%), but may have undergone accelerated bone thickening in the early stages.28 This explains why a relatively reduced volume of new bone formation was measured using micro-CT in the collagen group compared with the control group at 2 weeks.
At 4 weeks, the volume of new bone formation increased in the collagen membrane-treated group (8.26 mm3) compared with that observed at 2 weeks (1.73 mm3). This can also be explained by using data from the study of Gielkens et al.28 An increase in the defect closure rate would contribute to the volume of new bone generated after 2 weeks in the collagen membrane-treated group. At 4 weeks, a defect closure rate of 88.7% was measured in the collagen membrane-treated group and an increase in new bone volume of nearly five times was measured using micro-CT because new bone thickening could have accelerated with a simultaneous defect closure rate of almost 90%.28 At 8 weeks, we observed a minimal increase in newly formed bone in the collagen membrane-treated group (8.47 mm3). This is not surprising, because similar results have been shown in two in vivo studies that had a similar experimental design to ours. Gielkens et al.28 showed that there was an increase in the mean volume of new bone formed at 4 weeks (9.54 mm3) compared with that at 2 weeks (1.49 mm3), and van Leeuwen et al.21 showed a steep increase in the rate of bone formation between 2 and 4 weeks. In these studies, there was a minimal increase in bone formation observed between 4 and 12 weeks, which is similar to the pattern of new bone growth observed in our study.
The pattern of new bone formation in the silk fibroin membrane-treated group was not easy to understand. Unfortunately, microradiography data were not available for our study, and assumptions cannot be made easily based on previous experiments. If microradiographic analysis had been used in our study, then the growth patterns could have easily been understood. Despite having a similar ingrowth bone volume to the control group at 2 and 4 weeks, micro-CT analysis showed that there was no significant difference in the overall volume of bone formed at 8 weeks between the silk fibroin membrane- and collagen membrane-treated groups. It is possible that silk fibroin membranes may have a higher defect closure rate during the early period after implantation, as observed with the collagen membrane; this may contribute to the fact that no difference in the overall volume of bone formed was observed after 8 weeks between the silk fibroin membrane- and the collagen membrane-treated groups.
The rate of new bone formation can be affected by the biocompatibility and mechanical stability of the barrier membrane used.4,34 In addition, the extent and rate of degradation may influence the bone growth by changing the mechanical stability and biocompatibility of the membranes used.23 Biodegradation is a variable process and has a complex mechanism. Degradation usually follows four stages: hydration, loss of strength, loss of mass integrity, and degradation by cell phagocytosis.13 As biodegradable materials are usually grafted in hemorrhagic conditions, which are hydrophilic, the varying status of water solubility in the membrane can affect the extent of hydration and lead to a loss of strength and mass integrity.23 Host factors, such as the treatment site and the host immune system, can also affect degradation. Surgical skills and related medical conditions can also have an effect. Various factors can affect the rate of degradation, and this may be helpful in understanding the diverse bone growth patterns of each membrane. However, our study was unable to determine a clear difference in the degradation rate or its relationship to bone regeneration between the two membrane-treated groups. A better understanding of the action of silk fibroin membranes will promote the development of appropriately designed silk materials for medical applications,35 and further research to clarify these issues is required in the future.
Silk fibroin membranes successfully enhanced new bone generation in a rat calvarial defect model without any adverse inflammatory reactions. Similar volumes of bone regeneration were observed when compared with the use of collagen membranes. Considering the lower cost and zero risk of infectious transmission from animal tissue, silk fibroin membranes are good candidates as an alternative to the widely used collagen membranes in GBR.
Figures and Tables
Fig. 1
Rat calvarium showing (A) the bilateral 5 mm-sized bony defects and (B) calvarial defects covered by a silk fibroin membrane.
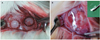
Fig. 2
Histological view of calvarial defects at 2, 4, and 8 weeks (original magnification ×40, hematoxylin and eosin stain). (A) photograph of Bio-Gide® membrane treated group at 2 weeks; (B) photograph of silk fibroin membrane treated group at 2 weeks; (C) photograph of control group at 2 weeks; (D) photograph of Bio-Gide® membrane treated group at 4 weeks; (E) photograph of silk fibroin membrane treated group at 4 weeks; (F) photograph of control group at 4 weeks; (G) photograph of Bio-Gide® membrane treated group at 8 weeks; (H) photograph of silk fibroin membrane treated group at 8 weeks; (I) photograph of control group at 8 weeks. BGM: Bio-Gide® membrane, SFM: silk fibroin membrane, OB: old bone, NB: new generating bone, IG: infiltrating growth of fibrotic and epithelial cells.
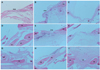
Fig. 3
Microcomputerized tomographic
image of the region of interest (ROI) in the
calvarial defect in the control, silk fibroin
membrane-, and Bio-Gide® membrane-treated
groups at 2, 4, and 8 weeks. The ROI is
denoted by the circled area in each image,
which shows the amount of new bone
generated.
Fig. 4
Graph of the mean volume (and SD) of new bone formation (in mm3) (y-axis) for the control, Bio-Gide®
membrane, and silk fibroin (SF) membrane-treated groups
at 2, 4, and 8 weeks after surgery.

Table 1
Absolute volume of new bone (in mm3) in the original defect as measured using micro-CT. The number of bony
defects in the control group was six at 2 weeks, seven in the Bio-Gide® group and the silk fibroin group at 2 and 4 weeks,
and eight in the other four groups

References
1. Becker BE, Becker W. Regeneration procedures: grafting materials, guided tissue regeneration, and growth factors. Curr Opin Dent. 1991; 1:93–97.
2. Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration-animal and human studies. Periodontol 2000. 1993; 1:26–35.
3. Hämmerle CH, Jung RE. Bone augmentation by means of barrier membranes. Periodontol 2000. 2003; 33:36–53.
4. Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988; 81:672–676.
5. Linde A, Thorén C, Dahlin C, Sandberg E. Creation of new bone by an osteopromotive membrane technique: an experimental study in rats. J Oral Maxillofac Surg. 1993; 51:892–897.
6. Hämmerle CH, Jung RE, Feloutzis A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J Clin Periodontol. 2002; 29:226–231.
7. Jung RE, Fenner N, Hämmerle CH, Zitzmann NU. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12-14 years. Clin Oral Implants Res. 2013; 24:1065–1073.
8. Dahlin C, Sennerby L, Lekholm U, Linde A, Nyman S. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1989; 4:19–25.
9. Davarpanah M, Tecucianu JF, Slama M, Celletti R. Bone regeneration in implantology. The use of Gore-Tex membranes: GTAM. J Parodontol. 1991; 10:169–176.
10. Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001; 72:512–516.
11. Simion M, Baldoni M, Rossi P, Zaffe D. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994; 14:166–180.
12. Schmidmaier G, Baehr K, Mohr S, Kretschmar M, Beck S, Wildemann B. Biodegradable polylactide membranes for bone defect coverage: biocompatibility testing, radiological and histological evaluation in a sheep model. Clin Oral Implants Res. 2006; 17:439–444.
13. Warrer K, Karring T, Nyman S, Gogolewski S. Guided tissue regeneration using biodegradable membranes of polylactic acid or polyurethane. J Clin Periodontol. 1992; 19:633–640.
14. Sevor JJ, Meffert RM, Cassingham RJ. Regeneration of dehisced alveolar bone adjacent to endosseous dental implants utilizing a resorbable collagen membrane: clinical and histologic results. Int J Periodontics Restorative Dent. 1993; 13:71–83.
15. Tams J, Rozema FR, Bos RR, Roodenburg JL, Nikkels PG, Vermey A. Poly(L-lactide) bone plates and screws for internal fixation of mandibular swing osteotomies. Int J Oral Maxillofac Surg. 1996; 25:20–24.
16. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004; 25:5821–5830.
17. Taylor MS, Daniels AU, Andriano KP, Heller J. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater. 1994; 5:151–157.
18. Zitzmann NU, Schärer P, Marinello CP. Long-term results of implants treated with guided bone regeneration: a 5-year prospective study. Int J Oral Maxillofac Implants. 2001; 16:355–366.
19. Fishman JA, Scobie L, Takeuchi Y. Xenotransplantationassociated infectious risk: a WHO consultation. Xenotransplantation. 2012; 19:72–81.
20. Pauli G. Tissue safety in view of CJD and variant CJD. Cell Tissue Bank. 2005; 6:191–200.
21. van Leeuwen AC, Huddleston Slater JJ, Gielkens PF, de Jong JR, Grijpma DW, Bos RR. Guided bone regeneration in rat mandibular defects using resorbable poly(trimethylene carbonate) barrier membranes. Acta Biomater. 2012; 8:1422–1429.
22. Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003; 24:401–416.
23. Cao Y, Wang B. Biodegradation of silk biomaterials. Int J Mol Sci. 2009; 10:1514–1524.
24. Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004; 25:1289–1297.
25. Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001; 54:139–148.
26. Kim KH, Jeong L, Park HN, Shin SY, Park WH, Lee SC, Kim TI, Park YJ, Seol YJ, Lee YM, Ku Y, Rhyu IC, Han SB, Chung CP. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J Biotechnol. 2005; 120:327–339.
27. Bosch C, Melsen B, Vargervik K. Importance of the criticalsize bone defect in testing bone-regenerating materials. J Craniofac Surg. 1998; 9:310–316.
28. Gielkens PF, Schortinghuis J, de Jong JR, Raghoebar GM, Stegenga B, Bos RR. Vivosorb, Bio-Gide, and Gore-Tex as barrier membranes in rat mandibular defects: an evaluation by microradiography and micro-CT. Clin Oral Implants Res. 2008; 19:516–521.
29. Rüegsegger P, Koller B, Müller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int. 1996; 58:24–29.
30. Dalstra M, Verna C, Cacciafesta V, Andreassen TT, Melsen B. Micro-computed tomography to evaluate bone remodeling and mineralization. Adv Exp Med Biol. 2001; 496:9–19.
31. Verna C, Dalstra M, Wikesjö UM, Trombelli L. Carles Bosch. Healing patterns in calvarial bone defects following guided bone regeneration in rats. A micro-CT scan analysis. J Clin Periodontol. 2002; 29:865–870.
32. Coelho PG, Giro G, Kim W, Granato R, Marin C, Bonfante EA, Bonfante S, Lilin T, Suzuki M. Evaluation of collagenbased membranes for guided bone regeneration, by three-dimensional computerized microtomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114:437–443.
33. Gielkens PF, Schortinghuis J, de Jong JR, Huysmans MC, Leeuwen MB, Raghoebar GM, Bos RR, Stegenga B. A comparison of micro-CT, microradiography and histomorphometry in bone research. Arch Oral Biol. 2008; 53:558–566.
34. Dahlin C, Andersson L, Linde A. Bone augmentation at fenestrated implants by an osteopromotive membrane technique. A controlled clinical study. Clin Oral Implants Res. 1991; 2:159–165.
35. Uebersax L, Hagenmüller H, Hofmann S, Gruenblatt E, Müller R, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 2006; 12:3417–3429.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download