Abstract
In the present study, we examined the effects of Dendropanax morbifera Léveille leaf extract (DML) on D-galactose-induced morphological changes in microglia and cytokines, including pro-inflammatory cytokines (interleukin [IL]-1β, IL-6, and tumor necrosis factor [TNF]-α) and anti-inflammatory cytokines (IL-4 and IL-10) in the hippocampus. Administration of DML to D-galactose-treated mice significantly improved D-galactose-induced reduction in escape latency, swimming speed, and spatial preference for the target quadrant. In addition, administration of DML to D-galactose-treated mice significantly ameliorated the microglial activation and increases of IL-1β, IL-6, and TNF-α levels in the hippocampus. Administration of D-galactose significantly reduced IL-4 levels in the hippocampus, while administration of DML to D-galactose-treated mice significantly increased IL-4 level. However, we did not observe any significant changes in IL-10 levels in hippocampal homogenates. These results suggest that DML reduces D-galactose-induced mouse senescence by reducing pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, as well as increasing anti-inflammatory cytokine IL-4.
Nervous tissue is more susceptible to oxidative damage than other tissue, because it has a high content of unsaturated fatty acids. Moreover, the brain has high metabolic activity and relatively low antioxidant defense [1]. In aging progresses, oxidative damage accumulates and the brain undergoes morphologic and functional changes [2]. Finally oxidative damage causes chronic inflammation in the aged brain. Neuroinflammation induces the activation of microglia in the brain and active microglia secrete pro-inflammatory cytokines (M1 microglia) or have important roles in brain repair and plasticity (M2 microglia) [3]. Activated microglia release neurotoxic substances and pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) [456]. In contrast, anti-inflammatory cytokines such as IL-4 and IL-10 may decrease neuroinflammation by regulating the production of IL-1β or IL-6 [7].
There are several animal models for the study of aging. However, the D-galactose model is the most convenient and can be compared to natural aging studies. Under normal condition, diet-fed D-galactose can be metabolized by two enzymes in the body: D-galactokinase and galactose-1-phosphate uridyl transferase. However, repeated treatment of D-galactose can be converted into hydrogen peroxide in the presence of galactose oxidase, leading to the formation of a superoxide anion [8]. Administration of D-galactose shows aging phenotypes such as impairment of spatial learning and memory, and neurodegeneration and neuroinflammation [8].
Dendropanax morbifera Léveille (D. morbifera) is an endemic species distributed in the southwest costal region and Jeju island of South Korea. Several lines of evidence demonstrate that D. morbifera leaf extract (DML) has antioxidant [91011] and anti-inflammatory [1213] effects. In particular, in our previous study, we demonstrated that D. morbifera stem extract ameliorates streptozotocin-induced memory impairment, microglial activation, and release of TNF-α and IL-1β in the hippocampus [13].
However, there are few reports on the effects of DML on age-related changes of hippocampal function. Therefore, in the present study, we evaluated the effects of DML on hippocampal function using a Morris water maze task, and microglial activation and subsequent cytokine release using immunohistochemistry and enzyme-linked immunosorbent assay (ELISA), respectively, in D-galactose treatment-induced aged mouse hippocampus.
Male C57BL/6 mice were purchased from Orient Bio Inc. (Seongnam, South Korea). Rats were housed in a conventional animal facility at 23℃ with 60% humidity, a 12-h/12-h light/dark cycle, and free access to food and tap water. The handling and care of the animals conformed to the guidelines established to comply with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). Ethical and experimental protocol approvals were obtained from the Institutional Animal Care and Use Committee (IACUC) of Kangwon National University (KW-161014-1). All of the experiments were conducted with an effort to minimize the number of animals used and the suffering caused by the procedures employed.
Fresh D. morbifera was supplied by HBJ Biofarm (Jeju, South Korea). The plant was identified by two practitioners of traditional Asian medicine. Leaves from the plant samples (100 g) were chopped, blended, soaked in 2 L of 80% ethanol, and then refluxed three times at 20℃ for 2 h. Insoluble materials were removed by centrifugation at 10,000×g for 30 min, and the resulting supernatant was concentrated and freeze-dried to yield a powder. Before each experiment, dried extracts were dissolved in distilled and deionized water.
Animals were divided into 3 groups (n=10 in each group): vehicle-treated, D-galactose-treated, and D-galactose with DML treated group. D-Galactose (100 mg/kg) and DML (100 mg/kg) were subcutaneously and orally administered to 7-week-old mice once a day for 10 weeks.
During the 10th week after D-galactose administration, spatial memory was assessed using a Morris water maze as described previously [14]. Morris water maze tests were performed in order to ensure objectivity in blind conditions. Three days after the training, the time individual mice spent to find the submerged platform (within 2 min) (escape latency) and the swimming distance were monitored by a digital camera and a computer system for 4 consecutive days during 4 trials per day. The administration of D-galactose and DML was continued during the water maze performance. For each trial, a mouse was placed in the water facing the wall at one of four starting positions and released. The swimming speed and the time required for the mouse to find the hidden platform were recorded via visual tracking system. The probe test was done on day 5; the platform was removed and the time that the mouse spent swimming in the target quadrant and in the three non-target quadrants (right, left, and opposite quadrants) was measured in the training and opposite quadrants for 60 s. In addition, the number of times the mouse crossed the platform site was recorded.
For immunohistochemical analysis, vehicle-treated, D-galactose-treated, and D-galactose with DML treated mice (n=5 per group) were anesthetized with 1 g/kg urethane (Sigma-Aldrich, St. Louis, MO, USA) and perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1M phosphate-buffer, pH 7.4. Brains were removed and postfixed in the same fixative for 24 h. Subsequently, the brain was dehydrated with graded concentrations of alcohol before being embedded in paraffin. Paraffin-embedded tissues were sectioned into 3-µm coronal sections using a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and were mounted onto silane-coated slides (Muto Pure Chemicals Co., Ltd, Tokyo, Japan).
To ensure that the immunohistochemical data was comparable among groups, sections were carefully processed under the same conditions. Sections were hydrated and treated with 0.3% hydrogen peroxide (H2O2) in phosphate-buffered saline (PBS) for 30 min. For antigen retrieval, the sections were placed in 400-mL jars filled with citrate buffer (pH 6.0) and heated in a 2100-retriever (Prestige Medical, Lancashire, UK). After antigen retrieval, slides were allowed to cool at room temperature and were washed in PBS. After washing, sections were incubated in 10% normal goat serum in PBS for 30 min. Sections were then incubated with rabbit anti-Iba-1 antibody (1:500, Wako, Osaka, Japan) for 48 h at 4℃. Sections were subsequently exposed to biotinylated goat anti-rabbit IgG, or anti-mouse IgG (diluted 1:200, Vector Laboratories, Inc., Burlingame, CA, USA), and streptavidin peroxidase complex (diluted 1:200, Vector Laboratories). Finally, sections were stained with 3,3-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, USA) in 0.1 M Tris-HCl buffer (pH 7.4).
Analysis of the regions of interest in the hippocampal CA1 region was performed using an image analysis system. Images were calibrated into an array of 512×512 pixels corresponding to a tissue area of 140 µm×140 µm (40× primary magnification). Pixel resolution was 256 gray levels. The intensity of Iba-1 immunoreactivity was evaluated by relative optical density (ROD), which was obtained after transformation of the mean gray level using the formula: ROD=log(256/mean gray level). The ROD of the background was determined in unlabeled portions (white matter region) and this value was subtracted to normalize the corrected optical densities using ImageJ 1.50 software (National Institutes of Health, Bethesda, MD, USA). The ratio of the ROD was calculated as percentage relative to control (vehicle-treated group).
To measure changes in TNF-α, IL-1β, IL-4, IL-6, and IL-10 levels in the hippocampus, vehicle-treated, D-galactose-treated, and D-galactose with DML treated mice (n=5) were sacrificed and used for ELISA. After sacrificing the mice and removing the hippocampus, hippocampal tissues were homogenized in ice-cold 50 mM sodium phosphate buffer (pH 7.4) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA) by using a glass-teflon homogenizer (Heidolph Silent Crusher M, Germany). The supernatant was separated by centrifugation at 20,000×g for 20min at 4℃. TNF-α, IL-1β, IL-4, IL-6, and IL-10 were measured in the supernatant of homogenized hippocampal tissue by using ELISA kits (EMD Millipore, Billerica, MA, USA). The procedures were carried out according to the manufacturer's instructions. TNF-α, IL-1β, IL-4, IL-6, and IL-10 levels were determined from a standard curve and were expressed in pg/mg protein.
Data are expressed as means for each experiment. The differences among the means were statistically analyzed by one- or two-way analysis of variance (ANOVA) with repeated measures and Bonferroni's post hoc test using GraphPad Prism 5.01 software (GraphPad Software, Inc., La Jolla, CA). Threshold for statistical significance was set to P<0.05.
Mean escape latency in the D-galactose-treated group was longer than in the vehicle-treated group on all days of experiments. However, differences were only significant on days 2, 3, and 4. Administration of DML to the D-galactose-treated group resulted in shorter mean escape latencies compared to escape latencies in the D-galactose-treated group. Differences were statistically significant on days 2 and 4. There was no significant difference in escape latency between the vehicle-treated group and D-galactose-treated group with DML (Figure 1A).
Mean swimming speed tended to be decreased after successive trials. In addition, mean swimming speed was slower in the D-galactose-treated group than in the vehicle-treated group. Administration of DML to the D-galactose-treated group increased the swimming speed in all trials although this tendency was not statistical significant (Figure 1B).
Significantly fewer platform crossings in the probe trial were observed in the D-galactose-treated group compared to the vehicle-treated group. Administration of DML to the D-galactose-treated group significantly increased the frequency of crossing over the platform site relative to that in the D-galactose-treated group and similar to that in the vehicle-treated group (Figure 1C).
In the probe trial for the escape latency task, mice form the D-galactose-treated group took significantly longer to find the target platform location than mice from the vehicle-treated group. Administration of DML to the D-galactose-treated group significantly reduced the time mice required to find the target platform (Figure 1D).
In the vehicle-treated group, Iba-1-immunoreactive microglia were present throughout hippocampus. These microglia showed small cytoplasms and long processes (Figure 2A). In the D-galactose-treated group, the distribution pattern was similar to that in the vehicle-treated group. However, Iba-1 immunoreactive microglia showed hypertrophy of the cytoplasm with highly ramified processes (Figure 2B). In this group, Iba-1 immunoreactivity was significantly increased by 148.6% relative to the vehicle-treated group (Figure 2D). In the D-galactose-treated group with DML, only a few Iba-1 immunoreactive microglia had hypertrophied cytoplasm, while most cells had small cytoplasms (Figure 2C). In addition, Iba-1 immunoreactivity was significantly decreased in this group compared to that in the D-galactose-treated group (Figure 2D).
In the D-galactose-treated group, pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 significantly increased by 1.71–2.46 fold relative to the vehicle-treated group in hippocampal homogenates. Anti-inflammatory cytokine IL-4 was significantly decreased in the D-galactose-treated group by 59.7% relative to the vehicle-treated group. In contrast, administration of DML to the D-galactose-treated group significantly decreased TNF-α, IL-1β, and IL-6 levels and increased IL-4 levels in hippocampal homogenates. However, IL-10 level did not change significantly among the hippocampal homogenates of the different groups (Figure 3).
Mounting evidence suggests that, with advancing age, the brain atrophies and its capacity for plasticity decreases [15]. In addition, age-related deficits resemble the deficits observed following bilateral damage to the hippocampus [16]. In the present study, we investigated the effect of DML on D-galactose-induced impairment of hippocampal functions based on the Morris water maze task. The Morris water maze task has been generally accepted as the key method for studying the relationship between hippocampal function and spatial learning and memory in rodents [17]. Repeated exposure to D-galactose for 10 weeks significantly decreased the spatial learning ability of mice. Supplementation of D-galactose with DML ameliorated reduced spatial learning ability. This result is consistent with results from a previous study showing that long-term treatment with D-galactose induces learning and memory deficits in both water maze and step-down latency [18]. In addition, D. morbifera stem extracts significantly improved novel object recognition memory in cadmium-exposed rats [19]. In addition, ethyl acetate fraction of D. morbifera ameliorated high fat diet-induced memory impairment measured in Y-maze, passive avoidance, and Morris water maze tests [20].
Aging is closely related to microglial activation. We monitored the effects of DML on D-galactose-induced morphological changes in Iba-1-immunoreactive microglia. Administration of D-galactose for 10 weeks induced microglial activation indicated by morphological changes from small cytoplasm with thin and long processes to hypertrophied cytoplasm and processes. A flow cytometry study previously demonstrated that activated microglia labeled with CD86 and MHC II are more common in aged (22-month-old) mice than in young adult (4-month-old) mice [21]. In the present study, administration of DML to D-galactose-exposed mice significantly decreased microglia activation. This result is in line with a previous study showing that D. morbifera stem extract significantly reduces the morphological changes indicative of the activated form of microglia in streptozotocin-exposed hippocampi [13].
In the present study, we analyzed the pro- and anti-inflammatory cytokines because microglial activation is associated with cytotoxicity [47] and neuroprotection [22]. Exposure to D-galactose for 10 weeks significantly increased the levels of TNF-α, IL-1β, and IL-6 in hippocampal homogenates. An in vitro neuron-microglia co-culture study showed that activated microglia elevate TNF-α and IL-1β mRNA [23]. IL-1β, IL-6, and TNF-α are released in the Alzheimer brain during chronic inflammation [24]. In addition, IL-6 levels are higher in the hippocampus of aged mice than in juvenile and adult mice [25]. In the present study, the administration of DML to D-galactose-treated mice significantly reduced D-galactose-induced increases of TNF-α, IL-1β, and IL-6. These results suggest that DML reduces neuroinflammation in the hippocampus of D-galactose-exposed mice. This finding may be closely related to the age-related impairment of memory function, because increases of IL-1β or IL-6 have been shown to correlate with the tau-phosphorylation in the Alzheimer brain [26]. In addition, elevated IL-1β decreases long-term potentiation (LTP) sustainability [27]. Conversely, lowering hippocampal IL-1β using minocycline treatment partially rescues this impairment in LTP [28]. In addition, D. morbifera stem extract also alleviates streptozotocin-induced increases in TNF-α and IL-1β levels in hippocampal homogenates [13].
In the present study, we observed that exposure to D-galactose significantly decreases IL-4 levels, and slightly increases IL-10 levels in hippocampal homogenates of mice. Furthermore, we observed that administration of DML to D-galactose-exposed mice rescued of IL-4 levels in the hippocampal homogenates. This result suggests that DML significantly increases the anti-inflammatory cytokine IL-4, but not IL-10, to reduce the inflammatory responses in the hippocampus. IL-4 levels have been shown to be increased in aged brains with increased neuroinflammation and reduced LTP [29]. Induction of hippocampal IL-4 restored LTP in the aged rats [30] and in the adult brains exposed to amyloid-β protein [31]. However, there have been conflicting reports on age-related changes of IL-10 levels in the brain. Ye and Johnson [32] demonstrated that IL-10 levels are decreased in aged (24-month-old) mouse brains. This may contribute to the increased expression of IL-6 in aged mouse brains. In contrast, Petez et al [33] observed slightly increased IL-10 expression in the aged (18-month-old) hippocampus relative to the adult (4-month-old) hippocampus in C57BL/6 mice. Other groups have also demonstrated that activated microglia from aged mice showed higher levels of IL-10 than activated microglia from adult mice [34]. In the present study, we did not observe any significant changes in IL-10 levels in hippocampal homogenates of D-galactose-treated mice. The discrepancies may be associated with the analysis methods used to measure IL-10 levels.
In conclusion, DML significantly ameliorates signs of D-galactose-induced mouse senescence by decreasing pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and increasing anti-inflammatory cytokine IL-4 in hippocampus.
Figures and Tables
Figure 1
Escape latency training trials (A), average speed (cm/sec) (B), frequency of target crossing (C) and time spent in correct quadrant (D) of vehicle-treated, D-galactose-treated, and D-galactose-treated group with Dendropanax morbifera Léveille leaf extract (D-galactose+DML) in the Morris water maze task (n=10; a indicates a significant difference from the vehicle-treated group; b indicates a significant difference from D-galactose-treated group). Vales are means±standard errors of the mean (SEM).

Figure 2
Immunohistochemical staining for ionized calcium-binding adapter molecule 1 (Iba-1) in the hippocampal CA1 region of vehicle-treated (A), D-galactose-treated (B), and D-galactose-treated group with Dendropanax morbifera Léveille leaf extract (D-galactose+DML, C). Scale bar=100 µm. D: Relative optical densities (ROD) are expressed as a percentage of the value of vehicle-treated group corresponding to a tissue area of 400×400 µm in the hippocampal CA1 region per section of vehicle-treated, D-galactose-treated, and D-galactose+DML-treated mice (n=5; a indicates a significant difference from vehicle-treated group; b indicates a significant difference from D-galactose-treated group). Vales are means±standard errors of the mean (SEM).
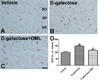
Figure 3
Levels of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, and anti-inflammatory cytokines such as IL-4 and IL-10 in hippocampi of vehicle-treated, D-galactose-treated, and D-galactose-treated group with Dendropanax morbifera Léveille leaf extract (D-galactose+DML) (n=5; a indicates a significant difference from vehicle-treated group; b indicates a significant difference from D-galactose-treated group). Vales are means± standard errors of the mean (SEM).

References
1. Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002; 1(2):117–123.
2. Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005; 81:1 Suppl. 313S–316S.
3. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009; 29(43):13435–13444.
4. Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T, Narita M. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010; 64(9):721–728.
5. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000; 51:245–270.
6. Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999; 54(7):M357–M364.
7. Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013; 39(1):19–34.
8. Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem Pharmacol. 2007; 74(7):1078–1090.
9. Kim W, Kim DW, Yoo DY, Jung HY, Nam SM, Kim JW, Hong SM, Kim DW, Choi JH, Moon SM, Yoon YS, Hwang IK. Dendropanax morbifera Léveille extract facilitates cadmium excretion and prevents oxidative damage in the hippocampus by increasing antioxidant levels in cadmium-exposed rats. BMC Complement Altern Med. 2014; 14:428.
10. Kim W, Kim DW, Yoo DY, Jung HY, Kim JW, Kim DW, Choi JH, Moon SM, Yoon YS, Hwang IK. Antioxidant effects of Dendropanax morbifera Léveille extract in the hippocampus of mercury-exposed rats. BMC Complement Altern Med. 2015; 15:247.
11. Kim ES, Lee JS, Akram M, Kim KA, Shin YJ, Yu JH, Bae ON. Protective activity of Dendropanax morbifera against cisplatin-induced acute kidney injury. Kidney Blood Press Res. 2015; 40(1):1–12.
12. Akram M, Kim KA, Kim ES, Syed AS, Kim CY, Lee JS, Bae ON. Potent Anti-inflammatory and Analgesic Actions of the Chloroform Extract of Dendropanax morbifera Mediated by the Nrf2/HO-1 Pathway. Biol Pharm Bull. 2016; 39(5):728–736.
13. Jung HY, Chung TH, Hwang IK. Dendropanax morbifera Léveille extract ameliorates memory impairments and inflammatory responses in the hippocampus of streptozotocin-induced type 1 diabetic rats. Mol Cell Toxicol. 2016; 12(4):429–436.
14. Yoo DY, Kim W, Lee CH, Shin BN, Nam SM, Choi JH, Won MH, Yoon YS, Hwang IK. Melatonin improves D-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res. 2012; 52(1):21–28.
15. Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012; 18(1):82–97.
16. Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003; 13(12):1344–1351.
17. Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981; 12(2):239–260.
18. Yu Y, Bai F, Wang W, Liu Y, Yuan Q, Qu S, Zhang T, Tian G, Li S, Li D, Ren G. Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol Biochem Behav. 2015; 133:122–131.
19. Kim W, Yim HS, Yoo DY, Jung HY, Kim JW, Choi JH, Yoon YS, Kim DW, Hwang IK. Dendropanax morbifera Léveille extract ameliorates cadmium-induced impairment in memory and hippocampal neurogenesis in rats. BMC Complement Altern Med. 2016; 16(1):452.
20. Kim JM, Park SK, Guo TJ, Kang JY, Ha JS, Lee DS, Lee U, Heo HJ. Anti-amnesic effect of Dendropanax morbifera via JNK signaling pathway on cognitive dysfunction in high-fat diet-induced diabetic mice. Behav Brain Res. 2016; 312:39–54.
21. Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation. 2013; 10:114.
22. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003; 3(1):23–35.
23. Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003; 23(5):1605–1611.
24. Gemma C, Bachstetter AD, Bickford PC. Neuron-Microglia Dialogue and Hippocampal Neurogenesis in the Aged Brain. Aging Dis. 2010; 1(3):232–244.
25. Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J Neuroimmunol. 2001; 117(1-2):87–96.
26. Quintanilla RA, Orellana DI, González-Billault C, Maccioni RB. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004; 295(1):245–257.
27. Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993; 628(1-2):227–234.
28. Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006; 99(4):1263–1272.
29. Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005; 26(5):717–728.
30. Clarke RM, Lyons A, O'Connell F, Deighan BF, Barry CE, Anyakoha NG, Nicolaou A, Lynch MA. A pivotal role for interleukin-4 in atorvastatin-associated neuroprotection in rat brain. J Biol Chem. 2008; 283(4):1808–1817.
31. Lyons A, Griffin RJ, Costelloe CE, Clarke RM, Lynch MA. IL-4 attenuates the neuroinflammation induced by amyloid-beta in vivo and in vitro. J Neurochem. 2007; 101(3):771–781.
32. Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001; 9(4):183–192.
33. Perez SD, Du K, Rendeiro C, Wang L, Wu Q, Rubakhin SS, Vazhappilly R, Baxter JH, Sweedler JV, Rhodes JS. A unique combination of micronutrients rejuvenates cognitive performance in aged mice. Behav Brain Res. 2017; 320:97–112.
34. Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009; 23(3):309–317.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download