Abstract
Artemisia argyi is used as a health supplement, tea, and food source in Korea. This study aimed to evaluate the effect of Artemisia argyi (AA) and its active compound, dehydromatricarin A (DA), on the attenuation of airway inflammation in a murine model of lipopolysaccharide (LPS)-induced acute lung injury (ALI). The C57BL/6 mice were administered AA (50 mg/kg or 100 mg/kg) and DA (10 mg/kg or 20 mg/kg) by oral gavage from day 0 to 7 days and LPS treated by intranasal instillation 48 hours before the sacrifice. The treatment of AA and DA markedly decreased inflammatory cells in the bronchoalveolar lavage fluid (BALF) compared with that in ALI-induced mice, which was accompanied by a significant reduction in the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in BALF. Furthermore, the administration of AA and DA clearly decreased inducible nitric oxide synthase (iNOS) expression and nuclear factor kappa B (NF-κB) phosphorylation in comparison with that in the ALI-induced mice. The histological examination of the lung tissue revealed that the administration of AA and DA suppressed the inflammatory cell infiltration into the peribronchial and alveolar lesions induced by LPS instillation. Collectively, our results indicated that AA and DA effectively decreased the airway inflammatory response induced by LPS instillation. Therefore, AA and DA may offer a potential therapy for airway inflammatory disease.
The persistent occurrence of acute lung injury (ALI) is a major clinical problem that leads to increased morbidity and mortality. ALI is characterized by pulmonary edema and severe acute inflammatory response; in particular, neutrophil accumulation results in dyspnea and death [1]. In the pathogenesis of ALI, excess neutrophil accumulation is facilitated by the complex interaction of inflammatory mediators [2]. Accumulated neutrophils can cause endothelial damage, enhance the permeability of the alveolar-capillary barrier, and release proinflammatory mediators, such as TNF-α and IL-6. Because these mediators exacerbate inflammatory responses, they are regarded as crucial markers of the acute phase of ALI [3456].
Lipopolysaccharide (LPS)-induced ALI animal models have been used to explore the mechanism of the pathogenesis of ALI and to investigate the therapeutic effects of the test material on ALI [7]. LPS, a major component of the cells wall of gram-negative bacteria, can induce pro-inflammatory cytokines and inflammatory cells [89]. LPS immediately stimulates the Toll-like receptor 4 (TLR4)-linked NF-κB pathway [10], which is related to not only a variety of physiological processes, but also to the development and progression of many inflammatory diseases [11]. NF-κB is activated by various stimuli and leads to the upregulation of many inflammatory mediators, such as TNF-α and IL-6, and inducible nitric oxide synthase (iNOS), which eventually induces pathophysiological responses that result in tissue damage [612]. Previous studies have shown that a reduction in the inflammatory responses, with a decrease in inflammatory cytokine and iNOS expression, was closely associated with the NF-κB pathway in in vivo and in vitro [1213].
The mugwort Artemisia argyi is used as tea and food in Korea [14]. A. argyi is a traditional medicine for the treatment of patients with abdominal pain, dysmenorrhea, and inflammation [15]. Recently, it was reported that A. argyi reduced allergic and inflammatory responses and oxidative stress [1617]. These properties were considered to be related to the amounts of phenolic compounds in A. argyi [1417]. We also recently demonstrated that A. argyi alleviates lung inflammation in an allergic asthma model and that it is the activity of Dehydromatricarin A in A. argyi [18]. However, there have been no studies on the effects of A. argyi against lung inflammation in an LPS-induced ALI model.
Therefore, we investigated the therapeutic effects of A. argyi and its active compound, dehydromatricarin A, on airway inflammation in an LPS-induced ALI model. In this study, we evaluated the production and expression of inflammatory mediators in LPS-induced lung tissue to clarify the possible mechanisms of action of A. argyi and dehydromatricarin A.
The experimental animal model used was designed by Shin et al. [13]. Male C57BL/6 mice (20-25 g) were purchased from Samtako Co. (Osan, Korea) and used when 8 weeks old. The mice were divided into seven groups: NC group, non-induced (control); LPS group, LPS-induced ALI; ROF group, roflumilast (10 mg/kg, P.O.)+LPS-induced ALI; AA-50 and AA-100 groups, A. argyi (50 and 100 mg/kg, P.O. respectively)+LPS; DA-10 and DA-20 groups; dehydromatricarin A (10 and 20 mg/kg, P.O. respectively)+LPS-induced ALI. LPS was administered by intranasal instillation of 10 µg in 50 µL PBS on day 5. Roflumilast, AA and, DA were orally administered for 7 days. Roflumilast is a PDE4 inhibitor used to treatment COPD. PDE4 inhibitor are known to possess anti-inflammatory effect. Seehase et al. suggested that roflumilast reduced pro-inflammatory cytokines such as TNF-α in LPS-induced acute lung inflammation [19]. Therefore, we used roflumilast as a positive control. The experiment was designed in accordance with the Institutional Animal Care and Use Committee of the Chonnam National University.
BALF collection was performed as previously described [15]. The animals received a tracheostomy under anesthesia and an endotracheal tube was inserted into trachea. BALF was obtained by filling the lung two times with ice-cold PBS (total volume: 1.4 mL). To count the inflammatory cells, 100 µL of BALF was centrifuged onto slides and stained by Diff-Quik® staining reagent (IMEB Inc., Deerfield, IL). The obtained supernatant was tested for inflammatory cytokines, including TNF-α, IL-6, and IL-β, by using commercial ELISA kits (R&D System, CA, USA) in accordance with the manufacturer's instructions. A quantitative analysis of cytokines was determined by the measurement of the absorbance at 450 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
To measure inflammatory protein expression, the lung tissue was homogenized with tissue lysis buffer supplemented with protease inhibitor (Sigma-Aldrich, Carlsbad, CA, USA) and the protein concentration was determined by using Bradford reagent (Bio-Rad Laboratories). Immunoblotting was performed as previously described [15] with the following primary antibodies: anti-iNOS (Santa Cruz, Santa Cruz, CA, USA), antip-NF-êB (1:1000 dilution; Abcam, Cambridge, MA, USA), anti-NF-κB (Abcam), anti-β-actin (Cell Signaling, Danvers, MA, USA). The band intensities of inflammatory proteins were measured by Chemi-doc (Bio-Rad Laboratories) and the relative expression values were computed by using IMT i-Solution software (IMT i-solution Inc., Vancouver, BC, Canada).
The lung tissue was fixed in 10% (v/v) neutral buffered formalin. The tissues were embedded in paraffin, sliced into 4-µm sections, and stained with hematoxylin and eosin (H&E) solution to estimate inflammatory cell recruitment. Each slide was examined manually in a completely blinded manner using a light microscope (Leica, Wetzlar, Germany) equipped with 10× and 20× objective lenses and a 100× oil immersion lens. Images of 10 randomly selected non-overlapping areas per slide were captured with a digital camera (IMT i-Solution Inc.).
ALI-induced mice (ALI mice) showed a significantly elevated inflammatory cell count in BALF in comparison with normal, induced control mice (normal mice) (Figure 1). Among inflammatory cells, the neutrophil count in ALI mice was more increased than that in the normal mice. However, the inflammatory cell counts were decreased in AA-treated mice compared to that in ALI mice; the decrease was especially prominent at 100 mg/kg AA. Similarly, DA-treatment also clearly decreased the inflammatory cell count in comparison with that in the normal mice. AA (100 mg/kg) and DA (20 mg/kg) treatment resulted in a similar reduction in the inflammatory cell counts in BALF.
ALI mice significantly increased the level of TNF-α in BALF in comparison with that in normal mice (Figure 2A). AA treatment at 100 mg/kg significantly reduced the TNF-α level in BALF compared to that in the ALI mice; although a decrease was observed with 50 mg/kg AA, the difference was not statistically significant. The level of TNF-α was noticeably decreased in DA-treated mice compared to that in ALI mice. Similarly, the level of IL-6 in BALF was increased more in ALI mice than in the control mice (Figure 2B); however, AA and DA-treated mice exhibited markedly decreased IL-6 levels in comparison with the ALI mice, except for the 50 mg/kg DA treatment.
ALI mice showed a marked elevation of iNOS expression in lung tissue compared with normal mice (Figure 3A). However, AA-treated mice significantly decreased the iNOS expression in lung tissue compared with ALI mice. In addition, DA-treated mice showed a notable reduction in iNOS expression compared with ALI mice. With regard to NF-κB phosphorylation, ALI mice displayed a notable elevation in lung tissue in comparison with normal mice, whereas AA- and DA-treated mice showed a significant reduction compared with ALI mice (Figure 3B). Although 50 mg/kg AA-treatment decreased the NF-κB phosphorylation compared with the ALI mice, the difference was not significant.
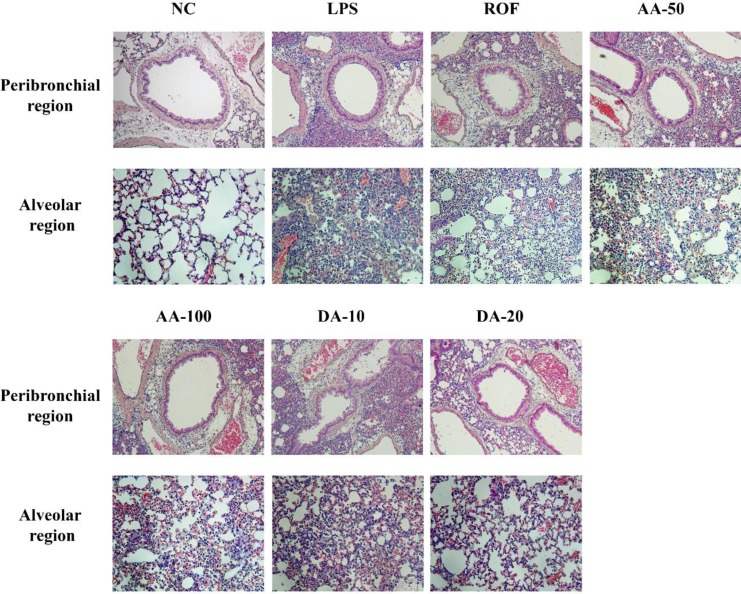
In the histological examination of the lung tissue, ALI mice showed extensive inflammatory cell infiltration into peribronchial and alveolar lesions in comparison with normal mice. However, AA-treated mice attenuated the histological alteration induced by LPS instillation. Additionally, DA-treated mice clearly reduced the inflammatory cell infiltration into lung tissue in comparison with ALI mice.
Acute lung injury (ALI) is a complex syndrome of intense pulmonary inflammatory response and the primary cause of death of intensive care patients [20]. In this study, we have explored the therapeutic effect of A. argyi (AA) and its active compound, dehydromatricarin A (DA), in an LPS-induced ALI mice model. AA-treatment in mice effectively suppressed the LPS-induced increases in inflammatory cell count and cytokines in BALF. AA also inhibited iNOS expression and NF-κB phosphorylation in ALI mice with a reduction in the inflammatory cell infiltration into lung tissue. Consistent with the results of AA-treatment, DA-treated mice also showed a significant reduction in inflammatory cell counts, cytokines, and pulmonary inflammation, which was accompanied by a reduction in iNOS expression and NF-κB phosphorylation.
ALI is characterized by the inflammatory responses that result from airway dysfunction and tissue remodeling in the respiratory tract. Inflammatory responses are regarded as an important defense system against exogenous harmful stimuli, but excess inflammation induces a loss of normal function and structural alterations owing to accumulated inflammatory cells and produces proinflammatory mediators, such as cytokines and chemokines [21]. In particular, among inflammatory cells, neutrophils are an essential component of the primary defense system; however, neutrophil accumulation into damaged lesions produced various proinflammatory cytokines and reactive oxygen species, which develop and aggravate ALI [22]. In this study, AA and DA treatment resulted in a significant reduction of the neutrophil count in BALF induced by LPS intranasal instillation. Furthermore, AA and DA treatment effectively attenuated the extensive inflammatory cell accumulation into lung tissue in ALI mice. These results indicated that AA has the potential to suppress neutrophil accumulation induced by LPS intranasal instillation.
TNF-α and IL-6 play key roles in the process of inflammation and stimulate the production of iNOS [23]. iNOS is a nitric oxide synthase (NOS) isoform; it is closely associated with NO production, which induces inflammatory responses [24]. In the pathogenesis of ALI, iNOS is considered a critical factor. Several studies have demonstrated that iNOS overexpression is related to the development and exacerbation of ALI owing to elevated NO and proinflammatory cytokine production in vivo and humans [2526]. In agreement with previous studies, LPS-induced ALI mice exhibited markedly increased TNF-α and IL-6 levels in BALF compared with the normal mice. However, AA and DA treatment significantly reduced the TNF-α and IL-6 levels by a decrease in iNOS expression in lung tissue in comparison with that in ALI mice. These results indicated that the therapeutic effects of AA may be related to the suppression of iNOS expression.
NF-κB is considered to be a limiting step in the inhibition of ALI development [252728]. In the inflammatory responses, NF-κB is activated by LPS stimulation and translocated into the nucleus via phosphorylation. Translocated NF-κB induces the transcription of inflammatory mediators, such as numerous cytokines, chemokines, and iNOS [2930]. Several reports have demonstrated that LPS-induced ALI increased the production of TNF-α and IL-6 and the expression of iNOS was increased via NF-κB signaling [63132]. Therefore, the suppression of NF-κB phosphorylation is considered an important therapeutic goal for the inhibition of ALI development. In this study, LPS-induced ALI mice clearly elevated NF-κB phosphorylation in comparison with the normal controls. In contrast, AA- and DA-treated mice significantly decreased NF-κB phosphorylation in comparison with ALI mice. These results indicated that the therapeutic effect of AA on ALI may be closely associated with the suppression of NF-κB phosphorylation.
In conclusion, our results showed that AA and DA effectively suppressed inflammatory cell count, cytokine production, and inflammatory cell accumulation in pulmonary inflammation in the LPS-induced ALI mice model. In addition, the effects of AA may be linked to the inhibition of NF-κB phosphorylation. Therefore, we suggested that AA has therapeutic potential for the treatment of LPS-induced acute lung injury.
References
1. Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007; 4(9):e269. PMID: 17803352.

2. Xiao Q, Dong N, Yao X, Wu D, Lu Y, Mao F, Zhu J, Li J, Huang J, Chen A, Huang L, Wang X, Yang G, He G, Xu Y, Lu W. Bufexamac ameliorates LPS-induced acute lung injury in mice by targeting LTA4H. Sci Rep. 2016; 6:25298. PMID: 27126280.

3. Yang SC, Chang SH, Hsieh PW, Huang YT, Ho CM, Tsai YF, Hwang TL. Dipeptide HCH6-1 inhibits neutrophil activation and protects against acute lung injury by blocking FPR1. Free Radic Biol Med. 2017; 106:254–269. PMID: 28232203.

4. Pedrazza L, Cunha AA, Luft C, Nunes NK, Schimitz F, Gassen RB, Breda RV, Donadio MV, de Souza Wyse AT, Pitrez PMC, Rosa JL, de Oliveira JR. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol. 2017; 232(12):3552–3564. PMID: 28112391.

5. Yingkun N, Zhenyu W, Jing L, Xiuyun L, Huimin Y. Stevioside protects LPS-induced acute lung injury in mice. Inflammation. 2013; 36(1):242–250. PMID: 22968433.

6. Al-Harbi NO, Imam F, Al-Harbi MM, Ansari MA, Zoheir KM, Korashy HM, Sayed-Ahmed MM, Attia SM, Shabanah OA, Ahmad SF. Dexamethasone Attenuates LPS-induced Acute Lung Injury through Inhibition of NF-κB, COX-2, and Pro-inflammatory Mediators. Immunol Invest. 2016; 45(4):349–369. PMID: 27104958.

7. Wang T, Hou W, Fu Z. Preventative effect of OMZ-SPT on lipopolysaccharide-induced acute lung injury and inflammation via nuclear factor-kappa B signaling in mice. Biochem Biophys Res Commun. 2017; 485(2):284–289. PMID: 28223218.

8. Wu Y, Jin F, Wang Y, Li F, Wang L, Wang Q, Ren Z, Wang Y. In vitro and in vivo anti-inflammatory effects of theaflavin-3,3'-digallate on lipopolysaccharide-induced inflammation. Eur J Pharmacol. 2017; 794:52–60. PMID: 27871911.

9. Zhang B, Liu ZY, Li YY, Luo Y, Liu ML, Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG, Li ZC. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur J Pharm Sci. 2011; 44(5):573–579. PMID: 22019524.

10. Yang S, Yu Z, Wang L, Yuan T, Wang X, Zhang X, Wang J, Lv Y, Du G. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activition. J Ethnopharmacol. 2017; 200:147–155. PMID: 28192201.

11. Shen B, Zhao C, Chen C, Li Z, Li Y, Tian Y, Feng H. Picroside II Protects Rat Lung and A549 Cell Against LPS-Induced Inflammation by the NF-κB Pathway. Inflammation. 2017; 40(3):752–761. PMID: 28161732.

12. Li N, Song Y, Zhao W, Han T, Lin S, Ramirez O, Liang L. Small interfering RNA targeting NF-κB attenuates lipopolysaccharideinduced acute lung injury in rats. BMC Physiol. 2016; 16(1):7. PMID: 28031043.

13. Shin NR, Shin IS, Song HH, Hong JM, Kwon OK, Jeon CM, Kim JH, Lee SW, Lee JK, Jin H, Li WY, Oh SR, Hahn KW, Ahn KS. Callicarpa japonica Thunb. reduces inflammatory responses: a mouse model of lipopolysaccharide-induced acute lung injury. Int Immunopharmacol. 2015; 26(1):174–180. PMID: 25662753.

14. Kim JK, Shin EC, Lim HJ, Choi SJ, Kim CR, Suh SH, Kim CJ, Park GG, Park CS, Kim HK, Choi JH, Song SW, Shin DH. Characterization of Nutritional Composition, Antioxidative Capacity, and Sensory Attributes of Seomae Mugwort, a Native Korean Variety of Artemisia argyi H. Lév. & Vaniot. J Anal Methods Chem. 2015; 2015:916346. PMID: 26550520.
15. Yun C, Jung Y, Chun W, Yang B, Ryu J, Lim C, Kim JH, Kim H, Cho SI. Anti-Inflammatory Effects of Artemisia Leaf Extract in Mice with Contact Dermatitis In Vitro and In Vivo. Mediators Inflamm. 2016; 2016:8027537. PMID: 27647952.
16. Lee NY, Chung KS, Jin JS, Bang KS, Eom YJ, Hong CH, Nugroho A, Park HJ, An HJ. Effect of Chicoric Acid on Mast Cell-Mediated Allergic Inflammation in Vitro and in Vivo. J Nat Prod. 2015; 78(12):2956–2962. PMID: 26593037.

17. Yao X, Wu D, Dong N, Ouyang P, Pu J, Hu Q, Wang J, Lu W, Huang J, Moracin C. A Phenolic Compound Isolated from Artocarpus heterophyllus, Suppresses Lipopolysaccharide-Activated Inflammatory Responses in Murine Raw264.7 Macrophages. Int J Mol Sci. 2016; 17(8):
18. Shin NR, Ryu HW, Ko JW, Park SH, Yuk HJ, Kim HJ, Kim JC, Jeong SH, Shin IS. Artemisia argyi attenuates airway inflammation in ovalbumin-induced asthmatic animals. J Ethnopharmacol. 2017; 209:108–115. PMID: 28735728.

19. Seehase S, Lauenstein HD, Schlumbohm C, Switalla S, Neuhaus V, Förster C, Fieguth HG, Pfennig O, Fuchs E, Kaup FJ, Bleyer M, Hohlfeld JM, Braun A, Sewald K, Knauf S. LPS-induced lung inflammation in marmoset monkeys-an acute model for antiinflammatory drug testing. PLoS One. 2012; 7(8):e43709. PMID: 22952743.
20. Yuan X, Wang Y, Du D, Hu Z, Xu M, Xu M, Liu Z. The effects of the combination of sodium ferulate and oxymatrine on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2012; 35(3):1161–1168. PMID: 22219049.

21. Kang P, Kim KY, Lee HS, Min SS, Seol GH. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013; 93(24):955–961. PMID: 24404587.

22. Han HJ, Li M, Son JK, Seo CS, Song SW, Kwak SH, Bae HB. Sauchinone, a lignan from Saururus chinensis, attenuates neutrophil pro-inflammatory activity and acute lung injury. Int Immunopharmacol. 2013; 17(2):471–477. PMID: 23928505.

23. Niu X, Hu H, Li W, Li Y, Huang H, Mu Q, Yao H, Li H. Protective effect of total alkaloids on lipopolysaccharide-induced acute lung injury. J Surg Res. 2014; 189(1):126–134. PMID: 24594217.

24. Park HJ, Jeon BT, Kim HC, Roh GS, Shin JH, Sung NJ, Han J, Kang D. Aged red garlic extract reduces lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages and acute pulmonary inflammation through haeme oxygenase-1 induction. Acta Physiol (Oxf). 2012; 205(1):61–70. PMID: 22353229.

25. Sun Q, Chen L, Gao M, Jiang W, Shao F, Li J, Wang J, Kou J, Yu B. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-κB. Int Immunopharmacol. 2012; 12(1):88–93. PMID: 22079591.

26. Zhang X, Huang H, Yang T, Ye Y, Shan J, Yin Z, Luo L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury. 2010; 41(7):746–752. PMID: 20227691.

27. Wu Q, Sun G, Yuan X, Soromou LW, Chen N, Xiong Y, Feng H. Tubeimoside-1 attenuates LPS-induced inflammation in RAW 264.7 macrophages and mouse models. Immunopharmacol Immunotoxicol. 2013; 35(4):514–523. PMID: 23844578.

28. Gao M, Chen L, Yu H, Sun Q, Kou J, Yu B. Diosgenin down-regulates NF-κB p65/p50 and p38MAPK pathways and attenuates acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol. 2013; 15(2):240–245. PMID: 23246979.

29. Chi G, Wei M, Xie X, Soromou LW, Liu F, Zhao S. Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation. 2013; 36(2):501–511. PMID: 23180366.

30. Chen L, Zhao L, Zhang C, Lan Z. Protective effect of p-cymene on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2014; 37(2):358–364. PMID: 24085645.

31. Ji MH, Li GM, Jia M, Zhu SH, Gao DP, Fan YX, Wu J, Yang JJ. Valproic acid attenuates lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2013; 36(6):1453–1459. PMID: 23846716.

32. Huang GJ, Deng JS, Chen CC, Huang CJ, Sung PJ, Huang SS, Kuo YH. Methanol extract of Antrodia camphorata protects against lipopolysaccharide-induced acute lung injury by suppressing NF-κB and MAPK pathways in mice. J Agric Food Chem. 2014; 62(23):5321–5329. PMID: 24849405.

Figure 1
In BALF, AA and DA decreased inflammatory cell counts. NC, non-treated mice; LPS, LPS-induced mice; ROF, roflumilast 10 mg/kg per day and LPS-induced mice; AA-50, A. argyi 50 mg/kg per day and LPS-induced mice; AA-100, A. argyi 100 mg/kg per day and LPS-induced mice; DA-10, dehydromatricarin A 10 mg/kg per day and LPS-induced mice; DA-20, dehydromatricarin A 20 mg/kg per day and LPS-induced mice. The values shown are the mean±SD. #P<0.05 vs NC; *P<0.05 vs LPS.
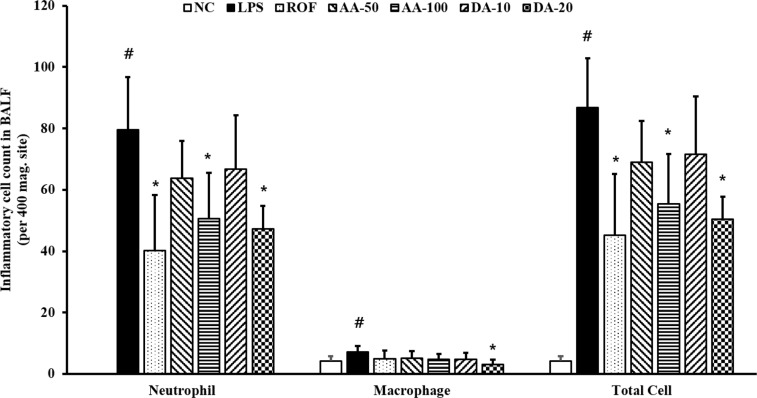
Figure 2
AA and DA reduced the production of inflammatory cytokines. (A) TNF-α, (B) IL-6. NC, non-treated mice; LPS, LPSinduced mice; ROF, roflumilast 10 mg/kg per day and LPS-induced mice; AA-50, A. argyi 50 mg/kg per day and LPS-induced mice; AA-100, A. argyi 100 mg/kg per day and LPS-induced mice; DA-10, dehydromatricarin A 10 mg/kg per day and LPS-induced mice; DA-20, dehydromatricarin A 20 mg/kg per day and LPS-induced mice. The values are shown are the mean ± SD. #P<0.05 vs NC; *P<0.05 vs LPS.
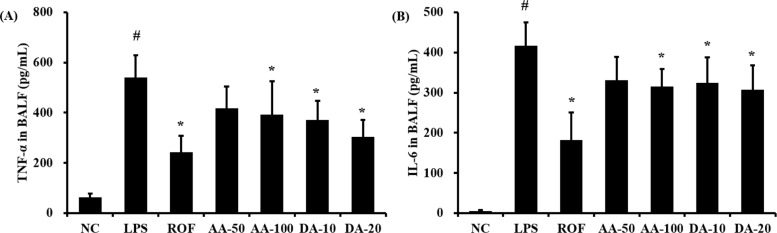
Figure 3
AA and DA decreased the expression of iNOS and phosphorylation of NF-κB. NC, non-treated mice; LPS, LPS-induced mice; ROF, roflumilast 10 mg/kg per day and LPS-induced mice; AA-50, A. argyi 50 mg/kg per day and LPS-induced mice; AA-100, A. argyi 100 mg/kg per day and LPS-induced mice; DA-10, dehydromatricarin A 10 mg/kg per day and LPS-induced mice; DA-20, dehydromatricarin A 20 mg/kg per day and LPS-induced mice. The values are shown as the mean±SD. #P<0.05 vs NC; *P<0.05 vs LPS.
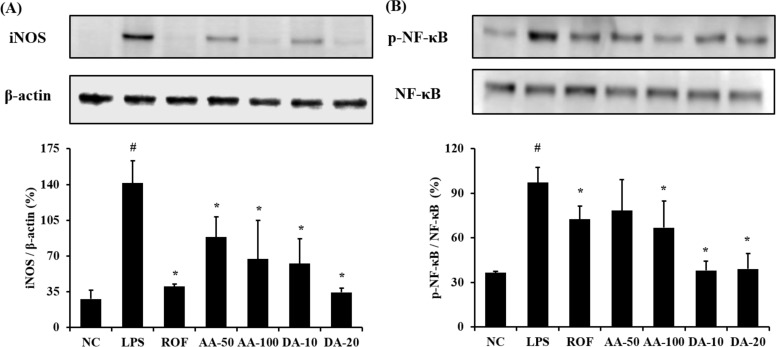
Figure 4
AA and DA attenuated inflammatory cell infiltration. NC, non-treated mice; LPS, LPS-induced mice; ROF, roflumilast 10 mg/kg per day and LPS-induced mice; AA-50, A. argyi 50 mg/kg per day and LPS-induced mice; AA-100, A. argyi 100 mg/kg per day and LPS-induced mice; DA-10, dehydromatricarin A 10 mg/kg per day and LPS-induced mice; DA-20, dehydromatricarin A 20 mg/kg per day and LPS-induced mice.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download