Abstract
Anticoccidial effects of the root bark of Dictamnus dasycarpus Turcz (Rutaceae) extract (DDE) were evaluated in chickens following oral infection with Eimeria (E.) tenella. Three-day-old chickens (n=30) were assigned to three groups (control, untreated, and DDE 0.1% treated). Chickens were fed a standard diet supplemented with or without DDE for 1 week prior to infection with E. tenella (10,000 sporulated oocysts per chicken). The effects of DDE on E. tenella infection were assessed by two parameters; fecal oocysts shedding and body weights gain. The DDE-fed chickens produced significantly reduced fecal oocysts (P<0.05) when compared to the E. tenella-infected group fed standard diet. Also, DDE-based diet, improved body weight loss caused by E. tenella infection. Our data demonstrated that DDE had remarkable anticoccidial activities against E. tenella. This finding might have implications for the development of anticoccidial drug. This study is the first to demonstrate anticoccidial effect of DDE on Eimeria parasites.
Coccidiosis is induced by Eimeria species infection and an important parasitic disease of poultry [1]. It is responsible for important economic losses in poultry production. E. tenella is important pathogen causing avian coccidiosis in laboratory avian animals and known to affect influencing experimental results obtained with contaminated animals [1,2]. The disease is characterized by enteric lesions of variable extent and severity, reducing the absorptive function of the intestinal mucosa, thus leading to weight loss, diarrhea, poorer feed conversion and a higher mortality in the affected flocks [3]. Losses include mortality, morbidity and cost of preventative or therapeutic drugs and/or vaccination. In addition, many of the in-feed medications commonly used for prevention of infections with Eimeria species have become less effective because some strains of parasites have developed reduced susceptibility to anticoccidials [4]. This suggests that coccidiosis is likely to have a greater impact on the profitability of broiler meat production in the future [4].
Dictamnus dasycarpus Turcz. (Rutaceae) is widely distributed in Asia, and root bark of this plant has been used for treatment of various ailments such as eczema, rubella, scabies, acute rheumatoid arthritis, jaundice, cold, and headache in Korean traditional medicine [5]. Also, it was reported the isolation and identification of six components inhibitory to the plant pathogenic fungus Cladosporium cucumerinum from the dichloromethane extract of D. dasycarpus [6]. It was found that a methanolic extract of the root bark of D. dasycarpus showed significant neuroprotective activity [5]. Moreover, its water extract was reported to inhibit the growth of many kinds of human pathogenic fungi in vitro [6]. Recently, the other effects of D. dasycarpus were also reported as anti-inflammation [7], insecticide [8], neuroprotection [5]. Known constituents of D. dasycarpus root bark include limonoids [5,6,9,10,11,12], furoquinoline alkaloids [12], flavonoids [10,11], coumarins [13], sesquiterpenes [14], sesquiterpene glycosides [14], and phenolic glycosides [15].
Although a variety types of natural products have been investigated in search for alternative controls of coccidiosis in chickens [1], the effects of Dictamnus dasycarpus on Eimeria infection has not been studied.
The present study is aimed to investigate the anticoccidial effects of Dictamnus dasycarpus (DDE) extract in chickens following oral infection with Eimeria (E.) tenella.
The dry mass of the root bark of Dictamnus dasycarpus Turcz was purchased from an Oriental Pharmacy (Iksan, Korea), was according to the standard as mentioned in Korean Pharmacopoeia and Korean Herbal Pharmacopoeia, which are the official compendia of standard. The procedure for preparing DDE was as follows. The air-dried mass of Dictamnus dasycarpus (100 g) was cut into pieces and extracted twice with 70% (v/v) ethanol (three times as much as the weight of the dried plants) for 3 h at 100℃. After filtration through a 400-mesh filter cloth, the filtrate was refiltered through filter paper (Whatman, No. 5) and concentrated on a rotary evaporator (EYELA, Tokyo, Japan) and the concentrated filtrate was evaporated to dryness under vacuum with freezing dryer (Labconco, USA). Finally, the solid residue was collected, placed in sealed bottles and stored at -20℃.
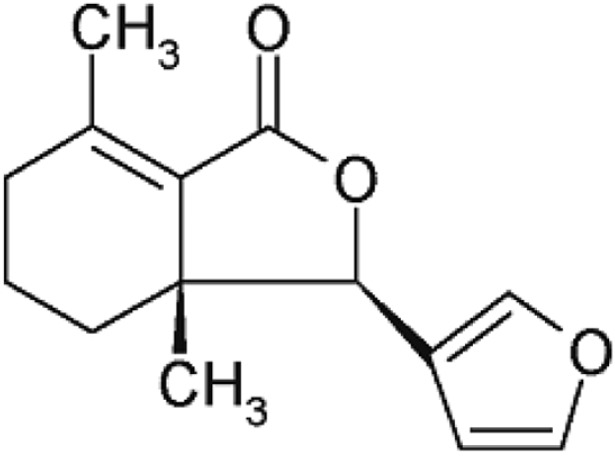
Fraxinellone, which was used for the standard material of DDE composition, was purchased from the Natural Product Bank, Institute for Korea Traditional Medical Industry (Geong-San, Korea). Figure 1 shows the chemical structures of fraxinellone. The Fraxinellone composition of DDE was analyzed by LC. Waters ACQUITY LC system (Waters Corp., Milford, USA) was used for LC system. The column was C18 type ACQUITY UPLC BEH (2.1×50 mm, 1.7 µm, Waters Corp., Milford, USA). A Waters Nova Pack C-18 column (ACQUITY UPLC BEH (2.1×50 mm, 1.7 µm, Waters Corp., Milford, USA) was employed. The wavelength of the UV detector was set at 300 nm. The column temperature was set at 30℃ with a flow rate of mobile phase at 0.6 mL/min (0.1% H3PO4/Acetonnitrile).
This study was conducted on the 3-day-old chickens (n=30) in the animal facility of Center for Animal Resources Development, Wonkwang University, Korea. Animals were acclimatized and kept in an animal facility room with regulated temperature (28±2℃), humidity (50±5%) and light/dark cycle (12/12 h). The animals were fed commercial post-broiler diet without antibiotics and coccidiostat (Hanil Feed Co., Yongin, Korea) and tab water ad libitum. The chickens were kept in wirefloored grower cages during study period. All studies were performed in accordance with the Guide for Animal Experimentation by Wonkwang University and approved by the Institutional Animal Care and Use Committee of Wonkwang University (WKU11-007). All efforts were made to minimize pain or discomfort of animals used.
Anticoccidial effects of DDE were evaluated in chickens following oral infection with E. tenella. Three-day-old chickens (n=30) were assigned to three groups (control, untreated, and DDE 0.1% treated). We decided the dose of DDE following as the preliminary results and the recommended concentration by feed companies. Chickens were fed a standard diet supplemented with or without DDE for 1 week prior to infection with E. tenella (10,000 sporulated oocysts per chicken). The effects of DDE on E. tenella infection were assessed by two parameters, fecal oocysts shedding and body weights gain.
Oocysts of E. tenella were cleaned by flotation on 5.25% sodium hypochlorite and washed three times with phosphate buffered saline. E. tenella was provided kindly by Professor Wongi Min at Gyeongsang National University in Korea. Chickens were treated orally by gavages using a 24 gauge, mouse stainless steel animal feeding tube (Popper & Sons, Inc., New York, USA) attached to a 3 mL syringe. The oral infectious dose of has been approximated 104 oocysts of E. tenella in 1 mL of saline. The control chickens (n=10) received saline through the same route.
During the study period, the animals were checked twice daily for morbidity and mortality. Further, we compared clinical signs and body weight changes of experimental animals. Body weights were individually measured for 2 weeks before infection and for 10 days post-infection.
Fecal materials were collected from 6 to 10 days postinfection. The fecal samples were analyzed for the presence of coccidial oocysts using a standard fecal flotation technique [16]. Briefly, 5 mL from each sample was pelleted by centrifugation at 1500×g for 5 min. The resulting pellet was resuspended in saturated sodium chloride (aqueous), passed through a 1 mm mesh size sieve to remove coarse fecal debris. The resulting filtrate was used in a standard gravity vial fecal flotation using 22 mm×22 mm coverslips. After flotation, the coverslip was mounted on a slide and examined in its entirety for the presence of coccidial oocysts. Total number of oocysts was calculated using the following formula: [total number of oocysts=oocyst count×dilution factor×(fecal sample volume/counting chamber volume)/number of birds per cage].
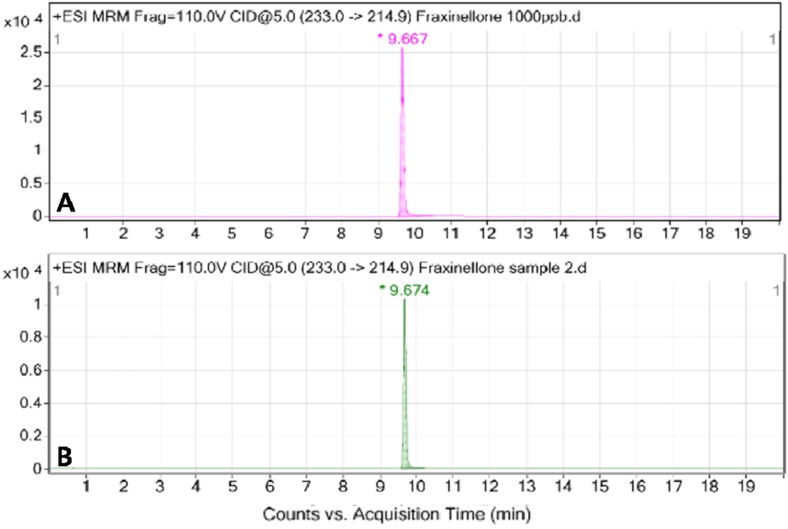
The extract yield of the dry root bark of Dictamnus dasycarpus Turcz with 70% ethanol was 16.86%. We analyzed DDE composition by LC. The retention time of fraxinellone in the specified LC condition was 9.667 min. The concentration of fraxinellone in DDE was 385 µg/mL. Figure 2 shows the LC chromatograph of DDE.
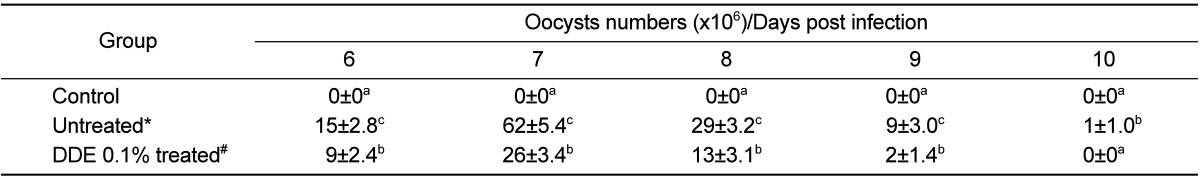
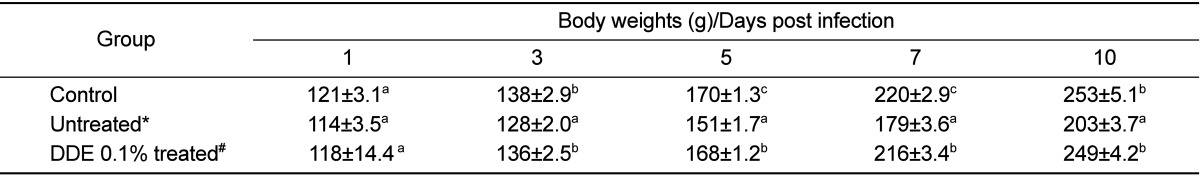
The effects of DDE on E. tenella infection were assessed by two parameters, fecal oocysts shedding and body weight gain. DDE 0.1% treated group produced significantly reduced fecal oocysts (P<0.05) when compared to the untreated group. Also, DDE-based diet, improved body weight loss caused by E. tenella infection.
The results showed that, compared to untreated group, DDE 0.1% treated group had significantly decreased fecal oocysts shedding and showed strong anticoccidial activities (P<0.01).
The results of this study showed that DDE had a strong anticoccidial effect on E. tenella. DDE contains a large amount of limonoids compounds. Fraxinellone, which is formed by the degradation of limonoids, has been reported to ameliorate infertility [17], to act as a vasorelaxant [18], to have neuroprotective properties [5], and to deter insects [19]. The limonoids are highly oxygenated terpenoids and have a range of pharmacological activities in man, for example, antibacterial, antifungal, antimalarial, anticancer, and antiviral effects [20].
Limonoids are described as modified triterpenes having a 4,4,8 trimethyl-17 furanyl steroid skeleton. Arrangements of subgroups and ring structures within this basic building block provide a host of characteristics that have generated interest in this plant product. These characteristics include insecticidal, insect growth regulation, insect antifeedant and medicinal effects to animals and humans such as antibacterial, antiviral, antifungal and anticarcinogenic properties [21]. Theses limonoid-included bioactive structures of DDE could be suggested a plausible explanation of the anticoccidial properties in this study.
In this study, anticoccidial effects of DDE were evaluated in chickens following oral infection with E. tenella. The DDE-fed chickens produced significantly reduced fecal oocysts (P<0.05) when compared to the E. tenella-infected group fed standard diet. Also, DDE-based diet, improved body weights loss caused by E. tenella infection. Our data demonstrated that DDE had remarkable anticoccidial activities against E. tenella. This finding might have implications for the development of anticoccidial drug. This study is the first to demonstrate anticoccidial effect of DDE on Eimeria parasites.
References
1. Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 2006; 5(1):143–163. PMID: 16451116.

2. McDougald LR. Protozoal infections. In : Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editors. Diseases of Poultry. Ames, USA: Iowa State Press;2003. p. 973–991.
3. Stotish RL, Wang CC, Meyenhofer M. Structure and composition of the oocyst wall of Eimeria tenella. J Parasitol. 1978; 64(6):1074–1081. PMID: 739302.
4. Williams RB. Relative virulences of a drug-resistant and a drugsensitive strain of Eimeria acervulina, a coccidium of chickens. Vet Parasitol. 2006; 135(1):15–23. PMID: 16361061.
5. Yoon JS, Sung SH, Kim YC. Neuroprotective limonoids of root bark of Dictamnus dasycarpus. J Nat Prod. 2008; 71(2):208–211. PMID: 18198838.
6. Zhao W, Wolfender JL, Hostettmann K, Xu R, Qin G. Antifungal alkaloids and limonoid derivatives from Dictamnus dasycarpus. Phytochemistry. 1998; 47(1):7–11. PMID: 9429316.
7. Kim H, Kim M, Kim H, Lee GS, An WG, Cho SI. Anti-inflammatory activities of Dictamnus dasycarpus Turcz., root bark on allergic contact dermatitis induced by dinitrofluorobenzene in mice. J Ethnopharmacol. 2013; 149(2):471–477. PMID: 23850712.
8. Lü M, Wu W, Liu H. Insecticidal and feeding deterrent effects of fraxinellone from Dictamnus dasycarpus against four major pests. Molecules. 2013; 18(3):2754–2762. PMID: 23455666.
9. Stoter R, Young DW. Preskimmianine: The Biogenetic Precursor of Skimmianine from Dictamnus albus L. Tetrahedron Lett. 1972; 13(22):2199–2202.
10. Souleles C. Flavonoids from Dictamnus albus. Planta Med. 1989; 55(4):402. PMID: 17262448.
11. Souleles C. A New Glavonoid Glycoside from Dictamnus albus. J Nat Prod. 1989; 52(6):1311–1312.
12. Stoter R, Young DW. Constituents of the root of Dictamnus albus L. Tetrahedron. 1973; 29(9):1217–1222.
13. Jeong SH, Han XH, Hong SS, Hwang JS, Hwang JH, Lee D, Lee MK, Ro JS, Hwang BY. Monoamine oxidase inhibitory coumarins from the aerial parts of Dictamnus albus. Arch Pharm Res. 2006; 29(12):1119–1124. PMID: 17225461.
14. Chang J, Xuan LJ, Xu YM, Zhang JS. Seven new sesquiterpene glycosides from the root bark of Dictamnus dasycarpus. J Nat Prod. 2001; 64(7):935–938. PMID: 11473427.
15. Chang J, Xuan LJ, Xu YM, Zhang JS. Cytotoxic terpenoid and immunosuppressive phenolic glycosides from the root bark of Dictamnus dasycarpus. Planta Med. 2002; 68(5):425–429. PMID: 12058319.
16. Lee HA, Hong S, Chung Y, Kim O. Sensitive and specific identification by polymerase chain reaction of Eimeria tenella and Eimeria maxima, important protozoan pathogens in laboratory avian facilities. Lab Anim Res. 2011; 27(3):255–258. PMID: 21998616.
17. Woo WS, Lee EB, Kang SS, Shin KH, Chi HJ. Antifertility principle of Dictamnus albus root bark. Planta Med. 1987; 53(5):399–401. PMID: 3432420.
18. Yu SM, Ko FN, Su MJ, Wu TS, Wang ML, Huang TF, Teng CM. Vasorelaxing effect in rat thoracic aorta caused by fraxinellone and dictamine isolated from the Chinese herb Dictamnus dasycarpus Turcz: comparison with cromakalim and Ca2+ channel blockers. Naunyn Schmiedebergs Arch Pharmacol. 1992; 345(3):349–355. PMID: 1377790.

19. Liu ZL, Xu YJ, Wu J, Goh SH, Ho SH. Feeding deterrents from Dictamnus dasycarpus Turcz against two stored-product insects. J Agric Food Chem. 2002; 50(6):1447–1450. PMID: 11879018.
20. Roy A, Saraf S. Limonoids: overview of significant bioactive triterpenes distributed in plants kingdom. Biol Pharm Bull. 2006; 29(2):191–201. PMID: 16462017.

21. Balestrieri E, Pizzimenti F, Ferlazzo A, Giofrè SV, Iannazzo D, Piperno A, Romeo R, Chiacchio MA, Mastino A, Macchi B. Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg Med Chem. 2011; 19(6):2084–2089. PMID: 21334901.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download