Abstract
Osteoporosis is a known major health problem and a serious disease of the bone, there has been a great need to develop more and newer animal models for this disease. Among animal models used for testing drug efficacy, the minipig model has become useful and effective due to its close similarity with humans (validity), particularly with the pharmacokinetics of compounds via subcutaneous administration, the structure and function of the organs, the morphology of bone and the overall metabolic nature. Based on these advantages, we sought to develop a new animal model of osteoporosis using micropig, which differs from other miniature pigs in the genetic background. Female micropigs were used for the induction of a moderate osteoporosis model by bilateral ovariectomy (OVX) and compared with shamoperated animals. For osteoporosis evaluation, clinical biomarkers such as blood osteocalcin (OSC) and parathyroid hormone (PTH) levels were measured, as well as bone mineral density (BMD) using micro-computed tomography (micro-CT). Compared to sham, OVX animals have decreased blood OSC level, while the blood PTH level increased in blood sera. In addition, we observed the significantly decreased BMDs of tibia region in OVX animals. Based on these results, we report that the micropig model developed in this study can be used to develop a new and effective medical method for diagnosis and treatment of osteoporosis.
The use of appropriate animal models for osteoporosis is important in understanding its pathogenesis and development, and also for testing of therapeutics in the treatment of this disease. Traditionally, rodent models have been used to study mechanism of osteoporosis. However there are also several drawbacks that rodents are not as close to humans genetically and physiologically, compared to other larger mammalian species. Among them, pigs share similar structure and function of organs, the development and morphology of the bones, and overall metabolism with humans [1-3], and have also demonstrated comparable pharmacokinetics of the compound via subcutaneous administration [4,5]; therefore the use of pigs shows a better alternative for replacement of rodents for use in osteoporosis research. Owing to their small size and ease of handling, minipigs were found to be suitable for use in long-term studies even at full maturity. These advantages regard the minipig of particular interest and best candidate for studies related to human osteoporosis [5-7]. Compared to surgical method, non-surgical method used by drugs, such as glucocorticoid, needs for long-term treatment over 15 month [4]. Postmenopausal osteoporosis is a major health problem for women and FDA requires preclinical studies in animal to evaluate agents [7]. Ovariectomy (OVX) has been adapted for establishment of animal models for osteoporosis, such as swine [6-8] and other animals [6,7,9,10,11]. However, there is no animal model that exactly mimics the postmenopausal osteoporosis.
There is a great need for testing and characterization of available animal models, and for induction procedures of osteoporosis models. In this study, in order to develop a new useful animal model for osteoporosis research, we performed osteoporosis induction in micropigs, which differ from other minipigs in genetic and physiologic characters. The micropig is a breed of laboratory swine developed and maintained since 2003 by the Medikinetics (MK) breeding program for biomedical research and nonclinical trials. The micropigs were a closed herd and classified two types of group, the tiny and medium according to growth rate and body weight.
A total of nine sexually matured female micropigs over six months of age were used in the present study. All animals were obtained from the closed barrier unit at Medikinetics (MK, Pyeongtaek, Korea) and were housed individually under controlled environmental conditions (temperature kept between 18 and 22℃, relative air humidity between 30-70%, with 15 air changes/h) and with a 12:12 hr light-dark cycle. Standard feeding regime was provided with pelleted diet sterilized by 2M rad radiation (Purina, Seongnam, Korea) and sterilized water ad libitum. Principles of laboratory animal care were followed in accordance with the Guide for Animal Experimental Protocol and this study was approved by the Institutional Animal Care and Use Committee of MK (MK-IACUC: 111125-001). Six female micropigs were anesthetized with isoflurane and their ovaries were removed bilaterally, according to the standard operation protocol of MK. The three remaining animals were sham-operated as control. After 6 weeks, osteocalcin (OSC) and parathyroid hormone (PTH) level, clinical biomarkers of osteoporosis, were measured in blood. Assay was performed in Samkwang Medical Laboratories (Seoul, Korea). Serum osteocalcin (ng/mL) was analysed using electrochemiluminescenceimmunoassay (ECLIA, Roche Diagnostics Corp., Basel, Switzerland), while serum parathyroid hormone (pg/mL) was measured using radioimmunoassay (RIA, Roche Diagnostics Corp., Basel, Switzerland).
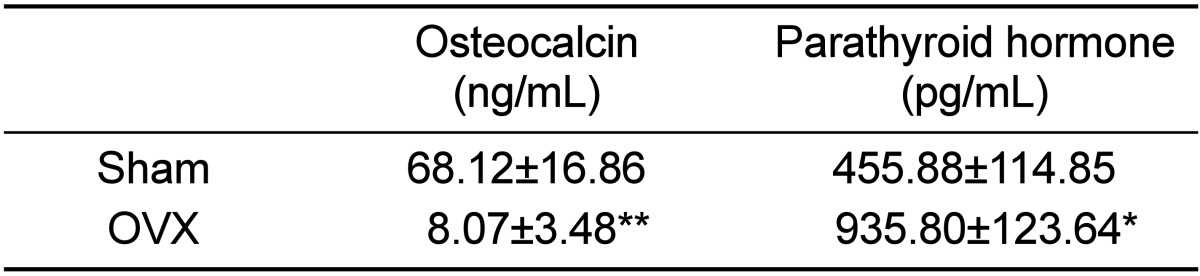
Ovariectomized micropigs that mimic the physiologic characteristics of osteoporosis in humans revealed moderately increased levels of PTH and decreased levels of OSC. Compared to sham animals, OVX micropigs showed significantly elevated levels of PTH (P<0.05) and decreased levels of OSC (P<0.01) (Table 1). These data indicate that the ovariectomy procedure used in this study resulted in the effective induction and maintenance of osteoporosis in micropigs, which is in agreement with findings from previous reports using other minipigs [12]. Lower levels of serum OSC were observed in micropigs, compared to sham, suggesting affected bone structures. As osteocalcin is produced by solely osteoblasts, decreased OSCs also indicates impaired osteoblast functions so this might result in imbalance of osteoblasts and osteoclasts followed by further loss of bone mass [13]. PTH enhances the release of calcium from the large reservoir contained in the bones, thus increased serum PTH indicates decalcification of bones.
To further confirm establishment of osteoporosis model, analysis of bone structures was performed using microCT scanning [14]. All micropigs were euthanized at 6 weeks post-ovariectomy/surgery, and the following specimens were harvested for sampling: the distal femur, proximal tibia and bodies of lumbar vertebrae (L3-L6). The bones were fixed in 10% neutralized buffered formalin, processed, and prepared for micro-CT imaging. Bone specimens were scanned by micro-CT (NFR Polaris-G90, NanoFocus Ray Co., JeonJu, Korea) equipped with a 4-±m focal spot microfocus X-ray tube as a source. The two-dimensional (2D) CT images were obtained at 70 kVp and 70 µAs and were reconstructed to 3D image in 512×512 pixel matrix. For quantitative analysis of the bone mineral density (BMD), ROIs (region of interest) were designated by using same protocols for each set of bones. The tibiae were scanned in an area between 30 and 32 mm from distal to proximal end, and a total of 13 micro-tomographic slices in 150-µm slice increments were obtained for each. L4 vertebral bodies were scanned in 2 mm from the caudal end of cranial growth plate, while the femurs in the area between 5 and 7 mm from the proximal end of lateral and medial epicondyles (Figure 1A,C,E). A total of 13 slices (about 2 mm) of samples were considered to be ROIs. After designating ROIs, thresholds were adjusted which is the standard value for separation of bony and non-bony materials (Figure 1D). For each sample, calculated standard thresholds were determined using computed programs for the analysis of BMDs. Measurements of BMDs were performed by NanoFocus Ray Co. (JeonJu. Korea).
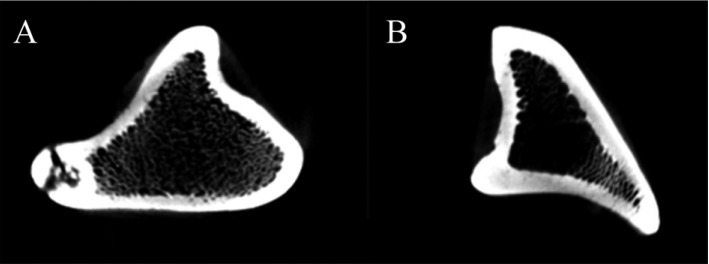
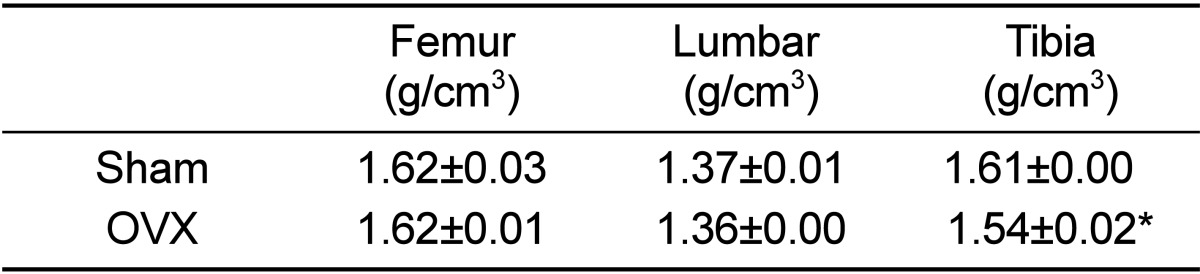
BMD in tibia was significantly decreased in OVX micropigs compared to sham (P<0.05) although not significantly evident in the femur and lumbar bones (Table 2). Cross section images of sham and OVX in tibia was showed (Figure 2). These results suggested that tibia area of bone in the micropigs was more sensitive to OVX compared with other areas. Tibia is the most commonly used region for analyzing BMDs [15], but femur and lumbar may also support in the clinical parameters of osteoporosis. Despite no significant change was observed on BMDs of femur and lumbar in the present study, the BMD of tibia and blood parameters were sufficient to indicate osteoporosis. Osteoporosis-related phenotypes by OVX in the micropigs, laboratory swine developed and maintained by the Medikinetics, was induced within a relatively short-period of time (Table 1, 2). More disease progression of osteoporosis pig model using OVX may be affected by additional factors, such as restriction of dietary calcium [10], treatment of immunosuppressive agents [4], and extension of experimental period [7].
In conclusion, based on the results described above, we hope that the micropig osteoporosis model can be used for developing effective candidate for postmenopausal osteoporosis and products for use in diagnosis and treatment of patients with the disease.
Acknowledgments
This research was supported by the technology innovation program (SV122684), the Small and Medium Business Administration, Korea.
References
1. Miller ER, Ullrey DE. The pig as a model for human nutrition. Annu Rev Nutr. 1987; 7:361–382. PMID: 3300739.

2. Hönig JF, Merten HA. Subperiosteal versus epiperiosteal forehead augmentation with hydroxylapatite for aesthetic facial contouring: experimental animal investigation and clinical application. Aesthetic Plast Surg. 1993; 17(2):93–98. PMID: 8390778.

3. Swindle MM, Smith AC. Comparative anatomy and physiology of the pig. Scand J Lab Anim Sci. 1998; 25:11–21.
4. Scholz-Ahrens KE, Delling G, Stampa B, Helfenstein A, Hahne HJ, Açil Y, Timm W, Barkmann R, Hassenpflug J, Schrezenmeir J, Glüer CC. Glucocorticosteroid-induced osteoporosis in adult primiparous Göttingen miniature pigs: effects on bone mineral and mineral metabolism. Am J Physiol Endocrinol Metab. 2007; 293(1):E385–E395. PMID: 17456640.

5. Akahoshi S, Sakai A, Arita S, Ikeda S, Morishita Y, Tsutsumi H, Ito M, Shiraishi A, Nakamura T. Modulation of bone turnover by alfacalcidol and/or alendronate does not prevent glucocorticoid-induced osteoporosis in growing minipigs. J Bone Miner Metab. 2005; 23(5):341–350. PMID: 16133683.

6. Jee WS, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact. 2001; 1(3):193–207. PMID: 15758493.
7. Turner AS. Animal models of osteoporosis--necessity and limitations. Eur Cell Mater. 2001; 1:66–81. PMID: 14562261.
8. Yoon KH, Cho DC, Yu SH, Kim KT, Jeon Y, Sung JK. The Change of Bone Metabolism in Ovariectomized Rats : Analyses of MicroCT Scan and Biochemical Markers of Bone Turnover. J Korean Neurosurg Soc. 2012; 51(6):323–327. PMID: 22949959.

9. Jochems C, Islander U, Erlandsson M, Verdrengh M, Ohlsson C, Carlsten H. Osteoporosis in experimental postmenopausal polyarthritis: the relative contributions of estrogen deficiency and inflammation. Arthritis Res Ther. 2005; 7(4):R837–R843. PMID: 15987485.
10. Mosekilde L, Weisbrode SE, Safron JA, Stills HF, Jankowsky ML, Ebert DC, Danielsen CC, Sogaard CH, Franks AF, Stevens ML, Paddock CL, Boyce RW. Calcium-restricted ovariectomized Sinclair S-1 minipigs: an animal model of osteopenia and trabecular plate perforation. Bone. 1993; 14(3):379–382. PMID: 8363881.

11. Boyce RW, Ebert DC, Youngs TA, Paddock CL, Mosekilde L, Stevens ML, Gundersen HJ. Unbiased estimation of vertebral trabecular connectivity in calcium-restricted ovariectomized minipigs. Bone. 1995; 16(6):637–642. PMID: 7669440.

12. Tsutsumi H, Katagiri K, Takeda S, Nasu T, Igarashi S, Tanigawa M, Mamba K. Standardized data and relationship between bone growth and bone metabolism in female Göttingen minipigs. Exp Anim. 2004; 53(4):331–337. PMID: 15297706.
13. Farrugia W, Fortune CL, Heath J, Caple IW, Wark JD. Osteocalcin as an index of osteoblast function during and after ovine pregnancy. Endocrinology. 1989; 125(3):1705–1710. PMID: 2788077.

14. Genant HK, Jiang Y. Advanced imaging assessment of bone quality. Ann N Y Acad Sci. 2006; 1068:410–428. PMID: 16831940.

15. Ikeda S, Morishita Y, Tsutsumi H, Ito M, Shiraishi A, Arita S, Akahoshi S, Narusawa K, Nakamura T. Reductions in bone turnover, mineral, and structure associated with mechanical properties of lumbar vertebra and femur in glucocorticoid-treated growing minipigs. Bone. 2003; 33(5):779–787. PMID: 14623053.

Figure 1
Micro-CT analysis of the femur, lumbar, and tibia bone of micorpig. 3D volume rendering images (A,C,E) and corresponding cross section images (B,D,F). (A) and (B), femur, (C) and (D), lumbar, (E) and (F), tibia. Green bars of A, C, E are designated ROIs. (E) Ulnar portion was excluded for ROI. (D) In lumbar's cross section, only red area (vertebral body portion) was calculated for BMD because other areas are included in transverse process and spinal process. Femur (B) and tibia (F) are calculated whole cross section. ROI; region of interest, BMD; bone mineral density.
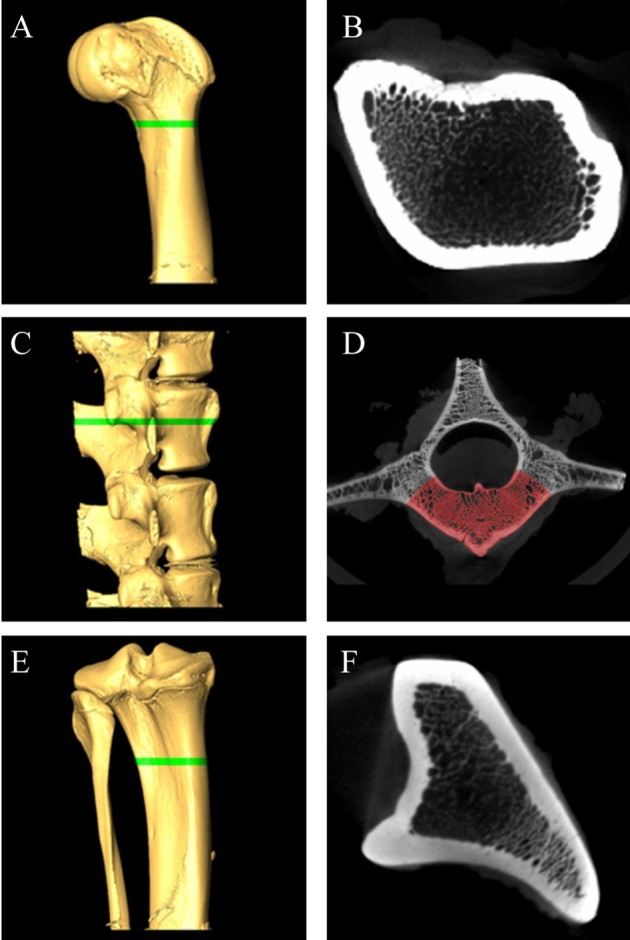
Figure 2
Corresponding cross section images of the tibia bone in sham operated (A) and ovariectomized (B) groups.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download