Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
Fig. 1
Dose of inhaled corticosteroids prescribed at the initiation date. The doses of ciclesonide and fluticasone are reported as actual doses; the beclomethasone doses were halved and thus reported as fluticasone-equivalents, as per recommendations regarding corticosteroid equivalence.1236

Fig. 2
Adjusted rate ratios (RRs) and odds ratios (ORs) for ciclesonide relative to fine-particle ICS during the 1 outcome year for the co-primary endpoints (severe exacerbation RR, risk domain asthma control OR, and overall asthma control OR) and secondary endpoints (change in therapy OR and daily short-acting β2-agonist [SABA] use OR).

Table 1
Demographic and clinical characteristics of the matched patients

Data are presented as n (%) unless otherwise stated.
GERD, gastroesophageal reflux disease; ICS, inhaled corticosteroid; IQR, interquartile range; LABA, long-acting β2-agonist; LTRA, leukotriene receptor antagonist; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist.
*Matching variable; †Evidence of rhinitis based on prescription of nasal corticosteroids during the baseline year or outcome analysis period; ‡Evidence of GERD/GERD prophylaxis based on prescriptions of proton-pump inhibitors during the baseline year or outcome analysis period; §Evidence of oral candidiasis at baseline, identified as topical oral antifungal prescription for oral candidiasis; ∥Acute oral corticosteroid prescription associated with asthma exacerbation treatment, defined as all courses that were definitely not maintenance therapy, AND/OR all courses where dosing instructions suggested exacerbation treatment (e.g. 6, 5, 4, 3, 2, 1 reducing, or 30 mg as directed), AND/OR all courses with no dosing instructions, but unlikely to be maintenance therapy, whereby maintenance therapy was defined as no evidence of reducing doses instructions, with a prescribed daily dose of <10 mg of prednisolone OR prescriptions of prednisolone tablets at a strength of 1 mg per day, with overall script coverage of more than 25% of days in a year; ¶Calculated as (count of inhalers×doses in pack/365)×µg strength; **P<0.01; ††P≤0.001.
Table 2
Outcome measures during a 1-year period after ICS initiation

Data are presented as n (%).
ICS, inhaled corticosteroid; SABA, short-acting β2-agonist
*Change in therapy during the outcome year was defined as an ICS dose increase of ≥50% or addition of new therapy including a leukotriene receptor antagonist, theophylline, or long-acting β2-agonist; †The controller-to-total medication ratio was defined as the number of controller units/(number of controller units+number of reliever units), where controllers included ICS and LTRA; ‡Antifungal medication definitely prescribed for treating oral candidiasis.
Table 3
Respiratory drugs prescribed during the outcome year
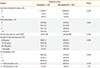
ICS, inhaled corticosteroid; IQR, interquartile range; LABA, long-acting β2-agonist; LTRA, leukotriene receptor antagonist; SABA, short-acting β2-agonist.
*ICS daily dose exposure calculated as (count of inhalers×doses in pack/365)×µg strength, using actual doses for ciclesonide and fluticasone-equivalent doses for fine-particle ICS, as per recommendations for corticosteroid equivalence.1236
ACKNOWLEDGMENTS
Notes
Study registration: European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP, study no. 6148).
The University of Groningen has received money for Professor Dirkje S. Postma regarding an unrestricted educational grant for research from Astra Zeneca, Chiesi. Travel to ERS and/or ATS has been partially funded by AstraZeneca, Chiesi, GSK, Takeda. Fees for consultancies were given to the University of Groningen by AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Takeda, and TEVA. Travel and lectures in China paid by Chiesi.
Richard Dekhuijzen has received over the past 3 years (i) fees for speaking, organising education, participation in advisory boards or consulting from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Mundipharma, Novartis, Pfizer, Teva, Zambon; (ii) research grants from Novartis, Boehringer Ingelheim, AstraZeneca.
Thys van der Molen has received grants for research, travel, reimbursement for presentations and advisory boards from AstraZeneca, GlaxoSmithKline, Almirall, Mundipharma, Boehringer Ingelheim, Chiesi, Teva and Novartis.
Richard J. Martin has done consultancy work and/or received travel support and/or honoraria for attendance at advisory boards for Teva, AstraZeneca, MedImmune, and Merck; received research grants from MedImmune and the NHLBI; received royalties from UpToDate.
Nicolas Roche has received over the past 3 years (i) fees for speaking, organising education, participation in advisory boards or consulting from Aerocrine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, MSD-Chibret, Mundipharma, Novartis, Pfizer, Stallergenes, Takeda, Teva; (ii) research grants from Novartis, Boehringer Ingelheim and Pfizer.
Wim van Aalderen is a member of Medical Advisory Board of Astra-Zeneca.
Theresa Guilbert reports personal fees from American Board of Pediatrics; Pediatric Pulmonary Subboard, grants and personal fees from Teva, personal fees from GSK and MERCK, personal fees from Regeneron Pharmaceuticals, grants from CDC, grants from DHHS, grants from NIH, grants from UW-Madison Medical and Education Research Committee, grants from Abbott Laboratories, grants from Array Biopharma, grants from Mylan, grants from Forest Research Institute, grants from F. Hoffman-LaRoche, grants from Medimmune, grants from KaloBios Pharmaceuticals, grants from Vertex Pharmaceuticals, grants from Roxane Laboratories and CompleWare Corporation, grants from CF Foundation Therapeutics, grants from Roche/Genetech, personal fees and other from Sanofi, personal fees and other from Novartis, and royalities from UpToDate.
Elliot Israel reports receiving consulting fees from AstraZeneca, Bird Rock Bio, Cowen & Co, Novartis, Nuvelution Pharmaceutical, Philips Respironics, Regeneron Pharmaceuticals, and TEVA Specialty Pharmaceuticals, and Vitaeris, Inc.; fees for expert testimony from Campbell, Campbell, Edwards & Conroy, Crammer, Bishop & O'Brien, Fox Rothschild, and Ryan Ryan Deluca LLP; travel grant support from Research in Real-Life (RiRL), TEVA Specialty Pharmaceuticals; royalties from UpToDate, Deputy Editor fees from the American Thoracic Society, DSMB Member for Novartis with no compensation, and having grant support paid to his Institution from Genentech, Sanofi, and the NIH.
Daniela van Eickels and Javaria Mona Khalid are employees of Takeda.
Ron M.C. Herings and Jetty A. Overbeek are employees of the PHARMO Institute. This independent research institute performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies.
Cristiana Miglio, Victoria Thomas, and Catherine Hutton are employees of Research in Real-Life (RiRL), which conducted this study and which has conducted paid research in respiratory disease on behalf of the following other organizations in the past 5 years: Aerocrine, AKL Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva, and Zentiva, a Sanofi company.
Elizabeth V. Hillyer is a consultant to RiRL and has received payment for writing and editorial support to Merck.
David B. Price has Board Membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva.
Consultancy: Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva, and Zentiva; Grants/Grants Pending with UK National Health Service, British Lung Foundation, Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva, and Zentiva; Payments for lectures/speaking: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda, and Teva; Payment for manuscript preparation: Mundipharma and Teva; Patents (planned, pending or issued): AKL Ltd.; Payment for the development of educational materials: GlaxoSmithKline, Novartis; Stock/Stock options: Shares in AKL Ltd which produces phytopharmaceuticals and owns 80% of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care; received Payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva; Funding for patient enrolment or completion of research: Almirral, Chiesi, Teva, and Zentiva; Peer reviewer for grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014); and Received unrestricted funding for investigator-initiated studies from Aerocrine, AKL Ltd, Almirall, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva, and Zentiva.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download