Abstract
Bamboo leaves (Phyllostachys pubescens Mazel ex J. Houz (Poacea)) have a long history of food and medical applications in Asia, including Japan and Korea. They have been used as a traditional medicine for centuries. We investigated the mechanism of anti-inflammatory activity of a bamboo leaf extract (BLE) on tumor necrosis factor-alpha (TNF-α)-induced monocyte adhesion in human umbilical vein endothelial cells (HUVECs). Exposure of HUVECs to BLE did not inhibit cell viability or cause morphological changes at concentrations ranging from 1 µg/ml to 1 mg/ml. Treatment with 0.1 mg/ml BLE caused 63% inhibition of monocyte adhesion in TNF-α-activated HUVECs, which was associated with 38.4% suppression of vascular cell adhesion molecule-1 expression. Furthermore, TNF-α-induced reactive oxygen species generation was decreased to 47.9% in BLE treated TNF-α-activated HUVECs. BLE (0.05 mg/ml) also caused about 50% inhibition of interleukin-6 secretion from lipopolysaccharide-stimulated monocyte. The results indicate that BLE may be clinically useful as an anti-inflammatory or anti-oxidant for human cardiovascular disease including atherosclerosis.
Endothelial cells are critically important for regulating flow through blood vessels, because they generate signaling molecules such as nitric oxide, prostacyclins, and endothelin. These compounds serve diverse autocrine and paracrine functions and are associated with the formation of an endothelial monolayer that provides a non-permeable barrier to protect the underlying vascular smooth muscle from circulating growth-promoting factors [1-2].
Many studies have reported that these blood vessel interactions are mediated by adhesion molecules. Cell adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion molecule-1 (ICAM-1), E-selectin, and P-selectin play key roles in several pathologies such as cardiovascular disorders (CVD), cancer, and inflammatory diseases [3-5]. The adhesion molecules mediate different monocyte migration steps from the bloodstream toward plaque-forming foci [6]. Monocyte interactions with the vascular endothelium include a multi-step consecutive process of binding flowing monocytes from the blood with subsequent monocyte rolling, arrest, firm adhesion, and ensuing diapedesis [7,8]. Atherosclerosis is a chronic inflammatory disorder of the arterial wall and the major cause of CVD. It is initiated by pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α). The inflammatory signals cause up-regulation of endothelial adhesion molecules and increase through an excess build-up of plaque on the inner wall of large blood vessels by the recruitment of monocytes, which ultimately disturbs blood flow [9].
Extensive epidemiological studies of atherosclerotic disease, a leading cause of death, have shown that consumption of natural dietary products is associated with a reduction in atherosclerosis and a protective effect [10-12]. Many studies have shown that extracts of natural products improve vascular endothelial dysfunction in both in vivo and in vitro atherosclerotic models [13,14]. Flavonoids, the main polyphenolic constituents in plant derived diets, are involved in nitric oxide-mediated vascular reactivity and restoration of endothelial function [13]. Root and rhizomes of Sweet flag have been also used as a remedy to treat various ailments including cardiovascular disease. A coronary vasodilator effect is due primarily to glycoside, alkaloid, and essential oil [14].
In accordance with reports about the phytochemical effects of natural products, bamboo leaves also have a long history of food and medical applications in Asia, including Japan and Korea. The effective ingredients in ethanol bamboo leaf extract (BLE) are flavonoids, phenolic acid, phytosterol, amino acids, and microelements. It has recently been revealed that bamboo leaves also have anti-oxidant and anti-tumor activities [15,16]. For example, a water extract of Sasa senanensis (Franchet et Savatier) Rehder (Poacea) leaves shows anti-tumor activity against mouse sarcoma S-180 cells and significantly induces the production of IL-2, IL-12, and the interferon-γ immune response [17]. BLE extracted from Phyllostachys pubescens Mazel ex J. Houz (Poacea) has been approved as a functional health food by the Korean Food and Drug Administration (KFDA; approval no. 2005-1) and has cholesterol and lipid lowering as well as anti-oxidative effects. However, the anti-inflammatory function of BLE in vascular endothelial cells is unknown.
We have focused on the anti-inflammatory action of BLE. In this study, we extended these findings and revealed that BLE significantly inhibits monocyte adhesion to TNF-α-activated human umbilical vein endothelial cells (HUVECs) and is associated with an inhibition of VCAM-1 expression.
HUVECs were used as endothelial cells in monocyte adhesion and adhesion molecule expression experiments [18]. HUVECs and monocyte U937 cells were obtained from Clonetics (Walkersville, MD, USA) and the American Type Culture Collection (Rockville, MD, USA), respectively. Endothelial growth medium (EGM-2), Dulbecco's Modified Essential Medium (DMEM), fetal bovine serum (FBS), and antibiotics were purchased from Gibco (Grand Island, NY, USA). Human TNF-α and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against VCAM-1 and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Sigma-Aldrich, respectively. An ELISA kit was purchased from R&D Systems (Minneapolis, MN, USA) to measure IL-6 in culture medium.
For preparation of BLE, bamboo (Phyllostachys pubescens) plants were washed and dried for 10 days at room temperature [19]. One kg of dried leaves was cut into small pieces, mixed with 10 L of 70% ethanol, and repeatedly heated three times at 80℃ for 7 h. The extracts were filtered through filter paper (Whatman, Florham Park, NJ, USA) and the filtrates were pooled and concentrated in a N-1000 rotary evaporator (Eyela, Tokyo, Japan) at 55-65℃ under reduced pressure, and then dried in a Speed Spec 3000 freeze dryer (Bio-Rad, Hercules, CA, USA) to obtain a standardized BLE (UniBEX™) [18]. The BLE contained friedelin (3.0%), isoorientin (0.52%), and β-sitosterol (0.24%) along with tricin (0.23%) and p-coumaric acid (0.50%) as marker compounds for standardization. A stock solution of BLE (100 mg/ml) was prepared in 50% dimethylsulfoxide (DMSO) and was diluted with fresh complete medium immediately before use.
HUVECs were grown and maintained in EGM2 and used between passages 3 and 6. Suspended U937 cell cultures were maintained in DMEM supplemented with 10% (v/v) FBS and antibiotics. Each cell line was incubated in an atmosphere of 95% air and 5% CO2 at 37℃. The effect of BLE on HUVEC viability was measured with an ADAM-MC automatic cell counter (Digital Bio, Seoul, South Korea), which analyzed propidium iodide (PI) staining.
Intracellular ROS generation was measured by fluorometric examination with dichlorofluoresceindiacetate (H2DCFDA). H2DCFDA is cleaved by nonspecific cellular esterases and oxidized in the presence of H2O2 and peroxidases to yield fluorescent 2',7'-dichlorofluorescein (DCF). Briefly, 1 × 105 cells were plated in a 12-well plate and allowed to attach overnight. The cells were activated with 15 ng/ml TNF-α and then treated with BLE in a concentration-dependent manner within 1 h. An equal volume of DMSO was added as a control. Subsequently, the cells were stained with 5 µM H2DCFDA for 30 min at 37℃. The cells were collected and fluorescence was analyzed using a Fluorskan (Thermo Scientific, Rockford, IL, USA) with a 485 nm excitation and a 530 nm emission filter set. Cells were treated with 100 µM H2O2 without BLE as a positive control.
The monocyte-endothelial cell adhesion assay was performed as described previously [19]. Briefly, U937 monocytes (1 × 107 cells/ml) were incubated with 1 µM 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxy-fluorescein acetoxymethyl ester (BCECF-AM) for 30 min at 37℃ in RPMI-1640 medium. HUVECs (3 × 104 cells/well) were seeded in 96-well plates to reach confluent monolayers and activated with 15 ng/ml TNF-α in EGM-2. The HUVECs were subsequently treated with BLE in a concentration-dependent manner for 18 h. The fluorescent labeled U937 monocytes were added to the activated HUVECs and incubated for a further 2 h. After washing out the unbound U937 three times, monocyte adhesion was measured by fluorescent intensity using a Fluoroskan (Thermo Scientific). Wells containing HUVECs alone were used as blanks.
IL-6 was assessed in the culture medium using a colorimetric ELISA kit, according to the manufacturer's instructions (R&D Systems). Three assays were performed at the desired time intervals to obtain reliable results. Cells in 96-well plates were stimulated with lipopolysaccharide (LPS) before being treated with 0.05 mg/ml BLE for 12 h. Then, 100 µl of culture supernatant was added to the biotinylated IL-6 antibody-coated wells. Following a 2 h incubation and washing, a streptavidin-horseradish peroxidase solution was serially added. Unbound streptavidin was washed out and the 3,3',5,5'-tetramethylbenzidine substrate was added. After 30 min, the reaction was stopped and the absorbance was measured at 450 nm.
Cells were treated with BLE at the specified concentrations for 18 h and lysed, as previously described [20]. The cell lysate was cleared by centrifugation at 12,000 × g for 20 min, and the supernatant was used for immunoblotting. Proteins were resolved by SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. Immunoblotting was performed using anti-VCAM-1 and the band intensity was quantified in a Epichem 3 Darkroom using LabWorks software (UVP, Upland, CA, USA) followed by normalization to the density of β-actin.
Differences in the measured variables between the control and BLE-treated groups were determined with a one-way analysis of variance in the Prism 4 software (GraphPad, La Jolla, CA) and followed by Dunnett's or Bonferroni's test for multiple comparisons. A P-value < 0.05 was considered significant.
To investigate potential BLE cell cytotoxicity, we examined the effect of BLE on HUVEC viability using PI staining. HUVEC viability was maintained consistently at 1 µg/ml-1 mg/ml of BLE. TNF-α stimulated HUVECs showed more than 90% viability at 1 mg/ml of BLE (Fig. 1A). HUVEC viability was not affected by exposure to BLE in a concentration-dependent manner compared with DMSO treated cells, indicating that BLE did not decrease HUVEC viability through nonspecific cytotoxicity. This result was also supported by the morphological images viewed under phase-contrast microscopy. As shown in Fig. 1B, HUVEC morphology was not affected by BLE treatment, even at 1 mg/ml, compared with that in vehicle-treated cells.
TNF-α stimulates ROS generation during the inflammation process [21]. Appropriately, we next determined the change in TNF-α-induced intracellular ROS generation after BLE treatment using a DCFDA fluorescent probe. An approximately 3.9-fold increase in ROS generation was observed in cells that were exposed to TNF-α, compared with basal levels. BLE-treated HUVECs exhibited a dose-dependent decrease in DCF fluorescence compared with that in vehicle-treated cells (Fig. 2A). For example, DCF fluorescence in HUVECs treated for 4 h with 0.1 and 1 mg/ml BLE decreased by about 40.8% and 52.1%, respectively, compared with that in the vehicle-treated cells (Fig. 2A). TNF-α-mediated ROS generation in HUVECs was effectively attenuated in the presence of BLE, as shown in Fig. 2B. These results suggest that BLE regulated the initiation of inflammation by inhibiting ROS generation in HUVECs.
We then determined the functional significance of BLE to monocyte adhesion in endothelial cells. Cells were pretreated with BLE and then incubated with TNF-α for 18 h. TNF-α-induced monocyte adhesion was significantly blocked in a BLE concentration-dependent manner, although monocyte adhesion was minimal in unstimulated endothelial cells. Exposure to 0.1 mg/ml BLE resulted in about a 63% decrease in monocyte adhesion relative to that in the vehicle-treated cells (Fig. 3A). The adhesion of monocytes to endothelial cells was fully inhibited to 15.3%. As shown in Fig. 3B, a significant inhibition of monocyte adhesion by BLE was also observed under fluorescent microscopy.
To further examine whether BLE mediated monocyte adhesion, which was revealed by a decrease in fluorescent intensity (Fig. 3B), we determined the effects of BLE on TNF-α-induced VCAM-1 expression on the HUVECs. Exposure of HUVECs to TNF-α induced a significant upregulation of VCAM-1 expression. Interestingly, BLE significantly inhibited TNF-α-induced cell expression of VCAM-1 in a concentration-dependent manner (Fig. 4A). For example, when incubated with 0.01 and 0.1 mg/ml BLE for 18 h, VCAM-1 expression was partially but significantly downregulated by 13.2% and 38.4%, respectively (Fig. 4B). Based on these results, it was confirmed that IC50 of BLE for monocyte adhesion was approximately 0.1 mg/ml.
Several cytokines including IL-6 are produced at inflammation sites and play a key role in the acute phase response. Cytokines also exert stimulatory effects on T- and B-cells; thus, favoring chronic inflammatory responses [22]. Recent studies have focused on whether natural agents significantly attenuate secretion and/or recruit cytokines to inflammation sites [23]. We performed an IL-6 specific ELISA to experimentally verify the inhibitory effect of BLE on IL-6 secretion in LPS-stimulated monocyte. As shown in Fig. 5, the level of IL-6 decreased about 50% in BLE-treated cells compared with that in BLE non-treated cells at 15 h after LPS stimulation. Additionally, this inhibitory effect was sustained until 20 h, although IL-6 secretion declined over a longer period. Collectively, these results indicate that BLE treatment decreased IL-6 secretion from LPS-stimulated monocytes for at least 20 h, supporting our result that the inflammatory process was prevented in BLE-treated endothelial cells.
In this study, we report that BLE has significant anti-inflammatory activity in TNF-α stimulated HUVECs. This conclusion is supported by the following observations: BLE caused a decrease in ROS levels, reduced VCAM-1expression in a concentration-dependent manner, and BLE-treated monocytes exhibited diminished IL-6 secretion. BLE did not affect cell viability even at 1 mg/ml, indicating that BLE is not cytotoxic.
Consumption of natural products is associated with a lowered risk of cardiovascular mortality [10-12]. Despite much evidence for the biological activity of natural agents, their precise mechanisms of action are not well studied. ROS play an important role in the regulation of cell adhesion [24]. In atherosclerosis, exposure of endothelial cells to chemokines or cytokines induces leukocyte adhesion and enhances VCAM and ICAM expression, both of which are mediated by the cellular generation of ROS [25-26]. Furthermore, studies using antioxidants strongly indicate that ROS are obligate leading signals for leukocyte infiltration into tissues, which depend on the expression of endothelial adhesion molecules [27]. In the present study, TNF-α-induced ROS generation in HUVECs decreased significantly by BLE in a concentration dependent manner. At concentrations of 0.1 and 1 mg/mL, BLE resulted in a 40.7% and 52.7% inhibition of ROS, respectively. Some studies about natural agents have also reported that antioxidants attenuate cytokine-induced adhesion molecule expression, indicating that increases in adhesion molecule expression may play a pivotal role in the progression of CVDs, including atherosclerosis [28-30]. We additionally found that BLE significantly prevented VCAM-1 expression induced by TNF-α stimulation. Treatment with 0.1 mg/ml of BLE markedly down-regulated TNF-α-induced VCAM-1 expression by 63.3%. In this respect, it is important that BLE has the ability to regulate adhesion molecule expression as potential drugs, which may be of therapeutic use in a variety of acute and chronic inflammatory diseases.
We also observed that BLE treatment resulted in a 50% decrease in IL-6 secretion from monocytes, suggesting that BLE may modulate U937 cell adhesion to TNF-α-stimulated endothelial cells by inhibiting the secretion of chemo-attractant molecules. These findings are consistent with previous reports indicating that natural anti-inflammatory agents have inhibitory effects on cytokine production, although the pro-inflammatory signaling may be evoked by different mechanisms. For example, the level of IL-1β-induced IL-6 protein release decreases in black tea extract-treated endothelial cells [31]. Also, the antioxidant agent α-tocopherol decreases the release of IL-1β from endothelial cells, resulting in down-regulation of monocyte proatherogenic activity [32].
TNF-α signaling during oxidative stress has been implicated in a central role in endothelial activation and several cardiovascular disorders [20]. In the present study, a possible mechanistic pathway for the anti-inflammatory role of BLE appeared to reduce TNF-α-induced VCAM-1 and monocyte adhesion by inhibiting ROS production. Furthermore, suppression of inflammatory cytokines such as IL-6 would contribute to the anti-inflammatory role of BLE in endothelial cells.
In conclusion, the present study demonstrates that BLE suppresses TNF-α-induced VCAM-1 expression by inhibiting oxidative stress and secretion of chemo-attractants in the vascular system. BLE may have anti-inflammatory effects on vascular inflammation and prevent the development of atherosclerosis.
Figures and Tables
Fig. 1
Bamboo leaf extract (BLE) treatment at 0.001-1 mg/ml did not reduce viability in human umbilical vein endothelial cells (HUVECs). (A) Effect of BLE treatment on viability of HUVECs. (B) HUVEC morphological changes in response to BLE (magnification, × 200). Data are presented as means ± SEMs (n = 3). *P < 0.05, significantly different compared with the control (DMSO) by one-way analysis of variance followed by Dunnett's test.
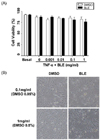
Fig. 2
Bamboo leaf extract (BLE) inhibited reactive oxygen species (ROS) generation in tumor necrosis factor-α (TNF-α)-stimulated human umbilical vein endothelial cells (HUVECs). (A) Fluorescence intensity of ROS in TNF-α-stimulated HUVECs following BLE treatment. Cells were labeled with the H2O2-sensitive fluorescent probe DCF-DA. (B) Representative fluorescent images show ROS levels in control cells and HUVECs stimulated with TNF-α in the absence or presence of BLE. Cells were observed under a fluorescent microscope at × 100 magnification. Data are presented as means ± SEMs (n = 3). *P < 0.05, significantly different compared with control (DMSO) by one-way analysis of variance followed by Dunnett's test.
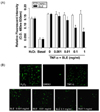
Fig. 3
Quantitative U937 monocyte adhesion assay in tumor necrosis factor-α (TNF-α)-stimulated human umbilical vein endothelial cells (HUVECs). (A) The intensity of fluorescence labeled-adherent U937 monocytes was measured with a fluorometer (ex 485/em 530 nm). (B) U937 cells adherent to HUVECs observed under a fluorescent microscope at × 100 magnification. Data are presented as means ± SEMs (n = 5). *P < 0.01 compared with TNF-α-treated group as determined by one-way analysis of variance followed by Dunnett's test.
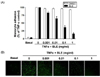
Fig. 4
Bamboo leaf extract (BLE) results in altered vascular cell adhesion molecule-1 (VCAM-1) expression in tumor necrosis factor-α (TNF-α)-stimulated human umbilical vein endothelial cells (HUVECs). (A) Immunoblotting for VCAM-1 using lysates from TNF-α-stimulated HUVECs treated with DMSO or BLE at the indicated concentrations. (B) Relative VCAM-1 expression was calculated based on densitometric scanning data of each band. Immunoblotting for each protein was performed at least twice using independently prepared lysates, and the results were consistent.
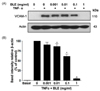
Fig. 5
Interleukin (IL)-6 secretion from lipopolysaccharide (LPS)-stimulated monocytes was partially but significantly attenuated by BLE. U937 monocytes were treated with LPS in the presence of 0.05 mg/ml BLE for the indicated time periods. IL-6 levels in the culture supernatant were measured by ELISA. Data are shown as means ± SEM (n = 3). *P < 0.05 compared to LPS alone as determined by one-way analysis of variance followed by Bonferroni's multiple comparison test.

References
1. Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction. Potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992. 85:1927–1938.

2. Harrison DG. Endothelial dysfunction in atherosclerosis. Basic Res Cardiol. 1994. 89:Suppl 1. 87–102.

3. Calabresi PA, Prat A, Biernacki K, Rollins J, Antel JP. T lymphocytes conditioned with Interferon beta induce membrane and soluble VCAM on human brain endothelial cells. J Neuroimmunol. 2001. 115:161–167.

4. James WG, Bullard DC, Hickey MJ. Critical role of the alpha 4 integrin/VCAM-1 pathway in cerebral leukocyte trafficking in lupus-prone MRL/fas(lpr) mice. J Immunol. 2003. 170:520–527.

6. Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001. 194:205–218.

7. McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001. 86:746–756.

8. Steeber DA, Tedder TF. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res. 2000. 22:299–317.

9. Wackers FJ, Soufer R, Zaret BL. Braunwald E, Zipes D, Libby P, editors. Nuclear cardiology. Heart Disease: A Textbook of Cardiovascular Medicine. 2001. 6th ed. Philadelphia: Saunders;288–290.
10. Campbell JH, Efendy JL, Smith NJ, Campbell GR. Molecular basis by which garlic suppresses atherosclerosis. J Nutr. 2001. 131:1006S–1009S.

11. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993. 342:1007–1011.

12. Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996. 312:478–481.

13. Grassi D, Aggio A, Onori L, Croce G, Tiberti S, Ferri C, Ferri L, Desideri G. Tea, flavonoids, and nitric oxide-mediated vascular reactivity. J Nutr. 2008. 138:1554S–1560S.

14. Shah AJ, Gilani AH. Aqueous-methanolic extract of sweet flag (Acorus calamus) possesses cardiac depressant and endothelialderived hyperpolarizing factor-mediated coronary vasodilator effects. J Nat Med. 2012. 66:119–126.

15. Kweon MH, Hwang HJ, Sung HC. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J Agric Food Chem. 2001. 49:4646–4655.

16. Park HS, Lim JH, Kim HJ, Choi HJ, Lee IS. Antioxidant flavone glycosides from the leaves of Sasa borealis. Arch Pharm Res. 2007. 30:161–166.

17. Seki T, Maeda H. Cancer preventive effect of Kumaizasa bamboo leaf extracts administered prior to carcinogenesis or cancer inoculation. Anticancer Res. 2010. 30:111–118.
18. Kim CS, Son SJ, Kim EK, Kim SN, Yoo DG, Kim HS, Ryoo SW, Lee SD, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 2006. 69:520–526.

19. Cho EJ, Park MS, Kim SS, Kang G, Choi S, Lee YR, Chang SJ, Lee KH, Lee SD, Park JB, Jeon BH. Vasorelaxing activity of Ulmus davidiana ethanol extracts in rats: activation of endothelial nitric oxide synthase. Korean J Physiol Pharmacol. 2011. 15:339–344.

20. Kim SN, Son SC, Lee SM, Kim CS, Yoo DG, Lee SK, Hur GM, Park JB, Jeon BH. Midazolam inhibits proinflammatory mediators in the lipopolysaccharide-activated macrophage. Anesthesiology. 2006. 105:105–110.

21. Weber KS, von Hundelshausen P, Clark-Lewis I, Weber PC, Weber C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur J Immunol. 1999. 29:700–712.

22. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006. 8:Suppl 2. S3.
23. Cao LH, Lee YJ, Kang DG, Kim JS, Lee HS. Effect of Zanthoxylum schinifolium on TNF-alpha-induced vascular inflammation in human umbilical vein endothelial cells. Vascul Pharmacol. 2009. 50:200–207.

24. Roy S, Sen CK, Packer L. Determination of cell-cell adhesion in response to oxidants and antioxidants. Methods Enzymol. 1999. 300:395–401.

25. Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA Jr. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985. 76:2003–2011.

26. Faruqi RM, DiCorleto PE. Mechanisms of monocyte recruitment and accumulation. Br Heart J. 1993. 69:S19–S29.

27. Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001. 280:C719–C741.

28. Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, Chen YL. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem. 2001. 82:512–521.

29. Hung CF, Huang TF, Chen BH, Shieh JM, Wu PH, Wu WB. Lycopene inhibits TNF-alpha-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur J Pharmacol. 2008. 586:275–282.

30. Murphy N, Grimsditch DC, Vidgeon-Hart M, Groot PH, Overend P, Benson GM, Graham A. Dietary antioxidants decrease serum soluble adhesion molecule (sVCAM-1, sICAM-1) but not chemokine (JE/MCP-1, KC) concentrations, and reduce atherosclerosis in C57BL but not apoE*3 Leiden mice fed an atherogenic diet. Dis Markers. 2005. 21:181–190.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download