Abstract
Dietary inorganic sulfur is the minor component in our diet, but some studies suggested that inorganic sulfur is maybe effective to treat cancer related illness. Therefore, this study aims to examine the effects of inorganic sulfur on cell proliferation and gene expression in MDA-MB-231 human breast cancer cells. MDA-MB-231 cells were cultured the absence or presence of various concentrations (12.5, 25, or 50 µmol/L) of inorganic sulfur. Inorganic sulfur significantly decreased proliferation after 72 h of incubation (P < 0.05). The protein expression of ErbB2 and its active form, pErbB2, were significantly reduced at inorganic sulfur concentrations of 50 µmol/L and greater than 25 µmol/L, respectively (P < 0.05). The mRNA expression of ErbB2 was significantly reduced at an inorganic sulfur concentration of 50 µmol/L (P < 0.05). The protein expression of ErbB3 and its active form, pErbB3, and the mRNA expression of ErbB3 were significantly reduced at inorganic sulfur concentrations greater than 25 µmol/L (P < 0.05). The protein and mRNA expression of Akt were significantly reduced at an inorganic sulfur concentration of 50 µmol/L (P < 0.05), but pAkt was not affected by inorganic sulfur treatment. The protein and mRNA expression of Bax were significantly increased with the addition of inorganic sulfur concentration of 50 µmol/L (P < 0.05). In conclusion, cell proliferation was suppressed by inorganic sulfur treatment through the ErbB-Akt pathway in MDA-MB-231 cells.
Sulfur is the seventh abundant mineral in body, and human weighing approximately 70 kg contains roughly 140 g of sulfur in body. Sulfur is consumed through food primarily in the form of sulfur-containing amino acids (SAAs), such as methionine, cysteine, cystine, and taurine, and in its glucosinolate form that is found in cruciferous vegetables, such as cabbage and cauliflower [1]. Sulfur is present in the sulfate form in water at a concentration of < 2 mg-> 1 g/L [1]. Moreover, sulfur can be ingested in inorganic forms, such as sulfite, sulfur dioxide, bisulfate, and met bisulfate, via preservatives that are added to processed food and drinks [2]. There is concern about toxicity of sulfur. Sulfide is produced when sulfur is incubated with the colon contents, and, as sulfide is associated with such symptoms as fatigue, drowsiness, and inhibited motor function [3]. However, toxicity due to inorganic sulfur is uncommon and appears to be restricted to the skin in the majority of cases [4]. When 0.17 mg/kg body weight of inorganic sulfur was orally administered to human, no toxicity was reported, nor was toxicity reported in animal tests using rabbits, rats, guinea pigs, and dogs [5]. The physiological effects of sulfur are mainly mediated by the organic form of sulfur. The anti-carcinogenic effects of organic sulfur were investigated via the sulfur-containing amino acids, (i.e., isothiocyanates, diallyl sulfide, allicin, glutathione, and α-lipoic acid) that are found in cruciferous plants, garlic, and methylsulfonylmethane (MSM), which is a byproduct of the wood pulp industry [6]. Sulfur is restrictively used in the treatment of dermatological disorders [3], psoriasis, rheumatic pain, and infections [7,8].
However, when inorganic sulfur is removed from the animal diet (even if there are sufficient SAAs for growth), avitaminosis E appears, collagen metabolism is damaged [9] and diet efficiency is reduced [10]. It is reported that pigs fed with high inorganic sulfur had an increase in SOCS3 mRNA in the ileum of pigs and thus likely counteracts the inflammatory activity of increased cytokine levels and minimizes cellular proliferation. High-S diet decreased the abundance of commensal bacteria with anti-inflammatory characteristics [11]. Inorganic sulfur is absorbed by active transport in the small intestines [12]. Inorganic sulfur also supplies the sulfur needed for cysteine formation and affects the functions of various physiological substances of which cysteine is a precursor [13].
Recently, it has been reported that the intake of refined inorganic sulfur reduces the clinical side effects of radiotherapy in cancer patients [14]. It was reported that when a daily dose of 0.5-1 g of refined sulfur was consumed in 24 cervical cancer patients, the sulfur, acting as a free radical scavenger, protected cells against DNA damage and reduced the side effects of radiotherapy. However, research on how inorganic sulfur can have anti-carcinogenic effects have not been well studied. Thus, we investigated the effect of inorganic sulfur on the inhibition of cell proliferation via regulation of epidermal growth factor receptor (EGFR) expression in MDA-MB-231 human breast cancer cells.
Inorganic sulfur powder with a purity of 99% or more was obtained from Sulfon PS, Inc. (Seoul, Korea). The inorganic sulfur powder was dissolved in methyl alcohol (Sigma Aldrich, St. Louis, MO, USA) at a concentration of 5 mmol and stored at -20℃. Dulbecco's modified Eagle's medium/Ham's F12 Nutrient Mixture (DMEM/F12) along with streptomycin and penicillin was obtained from Gibco/BRL (Grand Island, NY, USA). Antibodies against ErbB2, ErbB3, pErbB2, pErbB3, Bcl2, Bax, and pAkt were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and an antibody against Akt was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). RIA-grade bovine serum albumin (BSA), transferrin, and other reagents were purchased from Sigma (St. Louis, MO, USA).
MDA-MB-231 human breast cancer cells were purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were maintained in DMEM/F12 medium containing 100 mL/L of fetal bovine serum (FBS), 100,000 U/L of penicillin and 100 mg/L of streptomycin. The medium was replaced every 2-3 days. To examine of the effects of inorganic sulfur on breast cancer cell proliferation, the MDA-MB-231 cells were plated onto 24 well plates at a density of 2.5 × 104 cells/mL in DMEM/F12 medium supplemented with 10% FBS. After incubation for 48 h, the resulting cell monolayers were serum-starved with DMEM/F12 medium supplemented with 5 µg/mL of transferrin, 5 ng/mL of selenium, and 1 mg/mL of bovine serum albumin for 24 h. After serum starvation, the monolayers were incubated in serum-free medium (SFM) with 0, 12.5, 25, or 50 µmol/L of inorganic sulfur powder. The number of viable cells was estimated 24, 48, or 72 h after the cells were exposed to inorganic sulfur using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay as previously described [15]. The experiments were performed independently three times.
Cell lysates were prepared as previously described [16]. The total cell lysates were resolved on a 40-200 g/L sodium dodecyl sulfate polyacrylamide gel and were transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA). Next, the blot was blocked for 1 h in 10 g/L BSA in TBS-T (20 mmol/L of Tris-Cl, 150 mmol/L of NaCl, 1 g/L of Tween-20, pH 7.5) or 50 g/L of milk TBS-T after which it was incubated for 1 h with the appropriate antibody (ErbB2, pErbB2, ErbB3, pErbB3, Akt, p-Akt, Bcl2, or Bax). Next, the blot was incubated with an HRP-conjugated anti-mouse or anti-rabbit secondary antibody. The signals produced during the assay were detected using the enhanced chemiluminescence method and the Super-Signal West Dura Extended Duration Substrate (Pierce, Rockford, IL). Finally, the relative abundance of each protein band was analyzed by scanning the exposed films densitometrically using the Image J Launcher (provided by NCBI).
Total RNA was isolated using TRIzol reagent (Sigma), and cDNA was synthesized from 2 µg of total RNA using SuperScript II reverse transcriptase (lnvitrogen). To amplify the cDNA, primers for ErbB2 (upstream primer, 5'-CAAGAGTGCACGGCAGAGT-3'; downstream primer, 5'-GCCTTACAATGTGGGCATG-3'; annealed at 72℃ for 30 sec for 45 cycles), ErbB3 (upstream primer, 5'-CAAGAGTGCACGGCAGAGT-3'; downstream primer, 5'-GCCTTACAATGTGGGCATG-3'; annealed at 72℃ for 30 sec for 34 cycles), Akt (upstream primer, 5'-CAACTTCTCTGTGGCGCAGTG-3'; downstream primer, 5'-GACAGGTGGAAGAACAGCTCG-3'; annealed at 72℃ for 1 min for 30 cycles), Bcl2 (upstream primer, 5'-TGTGGATGACTGAGTACCTGAAC-3'; downstream primer, 5'-AGCTTTGTTTCATGGAACATCACTGAC-3'; annealed at 72℃ for 90 sec for 30 cycles), Bax (upstream primer, 5'-ATGGAGGGGTCCGGGGAG-3'; downstream primer, 5'-TGGAAGAAGATGGGCTGA-3'; annealed at 72℃ for 40 sec for 40 cycles) were used. The expression of human β-actin transcripts was examined as an internal control as described previously [17]. The PCR products were separated on a 1% agarose gel and were stained with ethidium bromide. The bands corresponding to each specific PCR product were quantified after the densitometric scanning of the exposed film using the Bio-Profile Bio-IL application (Vilber-Lourmat).
Statistical analyses were performed using the Statistical Analysis System software (SAS Institute, Cary, NC, USA). The data were expressed as means with their associated standard errors and analyzed with an analysis of variance (ANOVA). Any statistically significant differences among the means of the groups were tested at α = 0.05 using Duncan's multiple range test.
A MTT assay was performed to examine the effect of inorganic sulfur on the proliferation of cancer cells. Treatment with inorganic sulfur for up to 24 h did not affect cancer cell proliferation, but treatment for 72 h significantly decreased cell proliferation in a dose-dependent manner (P < 0.05) (Fig. 1).
The protein expression of ErbB2 and its active form, pErbB2, were significantly reduced following treatment with inorganic sulfur at concentrations of 25 µmol/L and 50 µmol/L, respectively (P < 0.05) (Fig. 2-A, 2-B). The protein expression of ErbB2 with the treatment of an inorganic sulfur concentration of 50 µmol/L was fell to 63.3% of the untreated control group. The protein expression of pErbB2 at the concentrations of 25 µmol/L and 50 µmol/L of inorganic sulfur was decreased to 13.1% and 2.4% of the untreated control group following treatment with, respectively. The mRNA expression of ErbB2 at a concentration of 50 µmol/L inorganic sulfur was significantly decreased to 37.2% (P < 0.05) of the level of the untreated control group (Fig. 2-C). The protein expression of ErbB3 significantly was decreased to 71.3% and 45.2% of the untreated control group following treatment with concentrations of 25 µmol/L and 50 µmol/L of inorganic sulfur, respectively (P < 0.05) (Fig. 3-A). The active form, pErbB3, was also significantly decreased to 47.2% and 30.3% of the untreated control group following treatment with concentrations of 25 µmol/L and 50 µmol/L of inorganic sulfur, respectively (P < 0.05) (Fig. 3-B). The mRNA expression of ErbB3 with the treatment of inorganic sulfur concentration of 25 µmol/L was also significantly decreased (P < 0.05) (Fig. 3-C). The protein and mRNA expressions of Akt were significantly decreased (P < 0.05) to 84.5% with the addition of 25 µmol/L inorganic sulfur and 62.4% of the level with the addition of 50 µmol/L, compared with untreated control group (P < 0.05) (Fig. 4-A, 4-C). However, the protein expression of pAkt was shown to be unaffected by treatment with inorganic sulfur (Fig. 4-B).
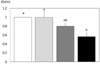
The protein and mRNA expression of Bax were significantly increased with the treatment of 50 µmol/L inorganic sulfur. The protein expression of Bax was significantly increased to 150.3% of the untreated control group following treatment with an inorganic sulfur concentration of 50 µmol/L (P < 0.05) (Fig. 5-A). The mRNA expression was also significantly increased to 398.7% of the untreated control group under the same conditions (P < 0.05) (Fig. 5-B). The protein and mRNA expression of Bcl2, a member of the Bcl family that inhibits cell apoptosis, were not affected by inorganic sulfur treatment (Fig. 6-A, 6-B). Consequently, the Bcl2/Bax protein expression ratio that is used as an indicator of apoptosis was significantly reduced to 56.4% of the ratio observed in the untreated control group (P < 0.05) following treatment with an inorganic sulfur concentration of 50 µmol/L (Fig. 7).
This study investigated the effects of inorganic sulfur on cancer cell proliferation and the protein and mRNA expression of proteins that are related to cell proliferation and apoptosis in MDA-MB-231 cells. There is concern about toxicity of sulfur. Toxicity by inorganic sulfur is usually limited to the skin [4], there is no toxicity was reported from oral administration of 0.17 mg/kg inorganic sulfur to humans, or in other animals [5].
Diverse instances of cancer cell proliferation inhibition due to treatment with organic sulfur have been reported [18-20], but the inhibition of breast cancer cell proliferation by inorganic sulfur is reported here for the first time. When treated with inorganic sulfur, there is no effect on cell proliferation following an incubation of up to 24 h, but cell proliferation was inhibited in a dose-dependent manner after 72 h. Kong et al. [18] showed that the treatment of AGS human gastric adenocarcinoma cells with extracts from young radishes that had a high organic sulfur glucosinolate content showed the cancer cell growth inhibition. In addition, Bak et al. [19] reported that treating HT-29 human colon cancer cells with Kimchi extract that made with sulfur-treated radishes likewise resulted in cancer cell growth inhibition. Choi and Kim [20] reported that, when diverse cancer cells were treated with extracts from a hot-water extraction from regular ducks or organic sulfur-fed ducks, a noticeable effect in proliferation inhibition was seen in the cells treated with the organic sulfur-fed duck extract. L-sulforaphane, the organic sulfur compound that is abundant in cruciferous plants, induces apoptosis in HT-29 colon cancer cells [21] and in PC-3 human prostate cancer cells [22].
The epidermal growth factor receptor (EGFR) family consists of ErbB1, ErbB2, ErbB3, and ErbB4. When these members combine with an outside ligand, they interact to form a heterodimer that activates the intercellular kinase domain, and an external signal is triggered inside the cell as the tyrosine portion of the receptor's cytoplasmic domain undergoes autophosphorylation. Of the ErbB members, ErbB3 alone forms a heterodimer with ErbB2 to activate the tyrosine kinase [23-25]. ErbB2 plays an important role in breast cancer cell development in rats [26], and when ErbB2 expression is high in the normal vascular epithelia near the carcinoma, there is a strong likelihood that the tissue will transform into cancer cells [27]. Therefore, it has been reported that the inhibition of excessive EGFR expression is closely related to the inhibition of cancer cell proliferation [28,29]. EGFR delivers an external signal to the cell through the PI3K/Akt pathway [23]. Akt inhibits the activation of pro-caspase-9 by inducing the phosphorylation of pro-caspase-9, and by inducing Bax and the pro-apoptotic transcription factor FKHR to undergo phosphorylation [30-32]. AKT promotes growth factor-based cell survival [33]. In this study, we showed that inorganic sulfur treatment significantly reduces the expression of the mRNA, protein and active forms of ErbB2 and ErbB3. Although, the protein expression of pAkt is not affected by treatment of inorganic sulfur, the protein and mRNA expression of Akt were similarly reduced. Therefore inorganic sulfur seems to play a role in inhibiting the proliferation of MDA-MD-231 cells by inhibiting the EGFR-Akt pathway partly.
Various apoptosis-related genes, such as Bcl2, Bax, p53, and Fas, are involved in the apoptosis of tumor cells [34,35]. Bcl2 is an important gene that inhibits cell apoptosis, and Bax is an apoptotic protein [23]. Bax is generally found in the cytoplasm but translocates into the mitochondria by apoptotic stimuli causing apoptosis by inducing mitochondrial dysfunction [36]. The stimulation of the Akt pathway stimulates cell survival by inhibiting the translocation of Bax into the mitochondria [37]. Our results indicate that inorganic sulfur treatment induces cell apoptosis by decreasing the Bcl2/Bax ratio by increasing the expression of Bax without affecting Bcl2. Because treatment with inorganic sulfur was shown to reduce the mRNA and protein expresssion of inactive Akt without affecting the levels of the active form of Akt, inorganic sulfur apparently affects the apoptosis of breast cancer cells at least partly through the EGFR-Akt-Bax pathway.
The aforementioned results show that inorganic sulfur inhibits cell proliferation by inhibiting the expression and activation of EGFR and by increasing the expression of Bax in MDA-MD-231 human breast cancer cells. More research is needed to investigate the independent anti-carcinogenic effects of inorganic sulfur, which differ from those of organic sulfur.
Figures and Tables
Fig. 1
Inorganic sulfur inhibited the cell proliferation in MDA-MB-231 cells. MDA-MB-231 cells were plated at a density of 2.5 × 104 cells/mL in a 24 well plate with DMEM/F12 supplemented with 10% FBS. Monolayers were then serum-starved with DMEM/F12 supplemented with 5 µg/mL transferrin, 5 ng/mL selenium, and 1 mg/mL bovine serum albumin for 24 h. After serum starvation, monolayers were incubated in serum-free medium with 0, 12.5, 25, or 50 µmol/L sulfur for 0, 24, or 72 h. Each bar represents the mean ± SE of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.

Fig. 2
Inorganic sulfur reduced the protein and mRNA expression of ErbB2 in MDA-MB-231 cells. For ErbB2 and pErbB2 protein expression, MDA-MB-231 cells were plated in a 100 mm dish at a density of 1 × 106 cells/dish with DMEM/F12 supplemented with 10% FDS for 48 h. The cells were then incubated in serum free medium for 24 h, after which they were then incubated in the presence of inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane and probed with ErbB2 (A) and pErbB2 (B). For ErbB2 mRNA expression, cells were cultured in serum-free medium with inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Total RNA was isolated and RT-PCR was performed (C). a) Photographs of the bands, which were representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± SE of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
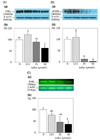
Fig. 3
Inorganic sulfur reduced the protein and mRNA expression of ErbB3 in MDA-MB-231 cells. For ErbB3 and pErbB3 protein expression, MDA-MB-231 cells were seeded in a 100 mm dish at a density of 1 × 106 cells/dish with DMEM/F12 supplemented with 10% FDS for 48 h. The cells were then incubated in serum free medium for 24 h, after which they were then incubated in the presence of inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane and probed with ErbB3 (A) and pErbB3 (B). For ErbB3 mRNA expression, cells were cultured in serum-free medium with inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Total RNA was isolated and RT-PCR was performed (C). a) Photographs of the bands, which were representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± SE of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
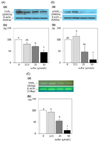
Fig. 4
Inorganic sulfur reduced the protein and mRNA expression of Akt MDA-MB-231 cells. For Akt and pAkt protein expression, MDA-MB-231 cells were seeded in a 100 mm dish at a density of 1 × 106 cells/dish with DMEM/F12 supplemented with 10% FDS for 48 h. Cells were then incubated in serum free medium for 24 h, after which they were then incubated in the presence of inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane and probed with Akt (A) and pAkt (B). For Akt mRNA expression, cells were cultured in serum-free medium with inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Total RNA was isolated and RT-PCR was performed (C). a) Photographs of the bands, which were representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± SE of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
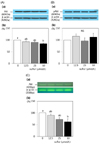
Fig. 5
Inorganic sulfur increased the protein and mRNA expression of Bax in MDA-MB-231 cells. For Bax protein expression, MDA-MB-231 cells were seeded in a 100 mm dish at a density of 1 × 106 cells/dish with DMEM/F12 supplemented with 10% FDS for 48 h. Cells were then incubated in serum free medium for 24 h, after which they were then incubated in the presence of inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane and probed with Bax (A). For Bax mRNA expression, cells were cultured in serum-free medium with inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Total RNA was isolated and RT-PCR was performed (B). a) Photographs of the bands, which were representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± SE of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.

Fig. 6
Inorganic sulfur did not influence the protein and mRNA expression of Bcl2 in MDA-MB-231 cells. For Bcl2 protein expression, MDA-MB-231 cells were seeded in a 100 mm dish at a density of 1 × 106 cells/dish with DMEM/F12 supplemented with 10% FDS for 48 h. Cells were then incubated in serum free medium for 24 h, after which they were then incubated in the presence of inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane and probed with Bcl2 (A). For mRNA expression, cells were cultured in serum-free medium with inorganic sulfur at concentrations of 0, 12.5, 25, or 50 µmol/L for 72 h. Total RNA was isolated and RT-PCR was performed (B). a) Photographs of the bands, which were representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± SE of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.

References
1. Ingenbleek Y. The nutritional relationship linking sulfur to nitrogen in living organisms. J Nutr. 2006. 136:1641S–1651S.

2. Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr. 2000. 72:1488–1494.

4. Sulfur. National Institute of Food and Drug Safety Evaluation [Internet]. 2010. cited 2010 August 10. Cheongwon: National Institute of Food and Drug Safety Evaluation;Available from: http://www.nifds.go.kr/toxinfo/Index.
5. Joo CN, Choi SD. Sulfur metabolism in animal body -absorption of sulfur from intestine and its distribution-. Korean Biochem J. 1992. 25:397–402.
6. Pappa A, Franco R, Schoneveld O, Galanis A, Sandaltzopoulos R, Panayiotidis MI. Sulfur-containing compounds in protecting against oxidant-mediated lung diseases. Curr Med Chem. 2007. 14:2590–2596.

7. Pratsel HG, Eigner UM, Weinert D, Limbach B. The analgesic efficacy of sulfur mud baths in treating rheumatic diseases of the soft tissues. A study using the double-blind control method. Vopr Kurortol Fizioter Lech Fiz Kult. 1992. (3):37–41.
8. Jung E. Sulfur, oil and tar-oil baths in geriatrics. Z Krankenpfl. 1971. 64:230–232.
9. Brown RG, Button GM, Smith JT. Changes in collagen metabolism caused by feeding diets low in inorganic sulfur. J Nutr. 1965. 87:228–232.

10. Button GM, Brown RG, Michels FG, Smith JT. Utilization of calcium and sodium sulfate by the rat. J Nutr. 1965. 87:211–216.

11. Kerr BJ, Weber TE, Ziemer CJ, Spence C, Cotta MA, Whitehead TR. Effect of dietary inorganic sulfur level on growth performance, fecal composition, and measures of inflammation and sulfate-reducing bacteria in the intestine of growing pigs. J Anim Sci. 2011. 89:426–437.

12. Anast C, Kennedy R, Volk G, Adamson L. In vitro studies of sulfate transport by the small intestine of the rat, rabbit, and hamster. J Lab Clin Med. 1965. 65:903–911.
13. Sasse CE, Baker DH. Sulfur utilization by the chick with emphasis on the effect of inorganic sulfate on the cystine-methionine interrelationship. J Nutr. 1974. 104:244–251.

14. Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002. 7:22–44.
15. Kim EJ, Kang IJ, Cho HJ, Kim WK, Ha YL, Park JH. Conjugated linoleic acid downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. J Nutr. 2003. 133:2675–2681.

16. Lee HS, Seo EY, Kim WK. Resveratrol induces apoptosis in SW480 human colon cancer cell lines. Food Sci Biotechnol. 2004. 13:80–84.
17. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003. 284:G996–G1005.
18. Kong CS, Bak SS, Rhee SH, Rho CW, Kim NK. Fermentation properties of young radish Kimchi prepared using young radish cultivated in the soil containing sulfur and it's inhibitory effect on the growth of AGS human gastric adenocarcinoma cells. J Korean Soc Food Sci Nutr. 2006. 35:158–163.

19. Bak SS, Kong CS, Rhee SH, Rho CW, Kim NK, Choi KL, Park KY. Effect of sulfur enriched young radish Kimchi on the induction of apoptosis in HT-29 human colon cancer cells. J Food Sci Nutr. 2006. 11:184–190.

20. Choi GH, Kim CH. Growth inhibition of extract from sulfur fed duck carcass against various cancer cell lines. J Korean Soc Food Sci Anim Resour. 2002. 22:348–351.
21. Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000. 60:1426–1433.
22. Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004. 25:83–90.

23. Takeuchi K, Ito F. EGF receptor in relation to tumor development: molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010. 277:316–326.

24. Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003. 12:541–552.

25. Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006. 7:505–516.

26. Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992. 89:10578–10582.

27. Han SW, Choi YY, Woo HD, Sohn DM, Bae SH, Gang GH, Kim SY, Baek MJ, Lim CW, Lee MS, Kim CH, Lee MH, Rho JH, Cho HD, Oh MH, Kim EH, Cho MS. Expression of HER-2/neu and paxillin in ductal carcinoma in situ, invasive ductal carcinoma with ductal carcinoma in situ and mucinous carcinoma. J Breast Cancer. 2008. 11:109–115.

28. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004. 101:13306–13311.

29. Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003. 9:2316–2326.
30. Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998. 282:1318–1321.

31. del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997. 278:687–689.

32. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999. 96:857–868.

33. Takeuchi K, Ito F. Suppression of adriamycin-induced apoptosis by sustained activation of the phosphatidylinositol-3'-OH kinase-Akt pathway. J Biol Chem. 2004. 279:892–900.

34. Binder C, Marx D, Binder L, Schauer A, Hiddemann W. Expression of Bax in relation to Bcl-2 and other predictive parameters in breast cancer. Ann Oncol. 1996. 7:129–133.

35. Keane MM, Ettenberg SA, Lowrey GA, Russell EK, Lipkowitz S. Fas expression and function in normal and malignant breast cell lines. Cancer Res. 1996. 56:4791–4798.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download