1. Padda MS, Picha DH. Quantification of phenolic acids and antioxidant activity in sweetpotato genotypes. Sci Hortic (Amsterdam). 2008; 119:17–20.

2. Yoshimoto M, Okuno S, Yoshinaga M, Yamakawa O, Yamaguchi M, Yamada J. Antimutagenicity of sweetpotato (Ipomoea batatas) roots. Biosci Biotechnol Biochem. 1999; 63:537–541.

3. Choi JH, Choi CY, Lee KJ, Hwang YP, Chung YC, Jeong HG. Hepatoprotective effects of an anthocyanin fraction from purple-fleshed sweet potato against acetaminophen-induced liver damage in mice. J Med Food. 2009; 12:320–326.

4. Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by d-galactose in mouse liver. Food Chem Toxicol. 2009; 47:496–501.

5. Vivas N, Lonvaud-Funel A, Glories Y. Effect of phenolic acids and anthocyanins on growth, viability and malolactic activity of a lactic acid bacterium. Food Microbiol. 1997; 14:291–300.

6. Hagiwara A, Yoshino H, Ichihara T, Kawabe M, Tamano S, Aoki H, Koda T, Nakamura M, Imaida K, Ito N, Shirai T. Prevention by natural food anthocyanins, purple sweet potato color and red cabbage color, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-associated colorectal carcinogenesis in rats initiated with 1,2-dimethylhydrazine. J Toxicol Sci. 2002; 27:57–68.

7. Hayashi K, Hibasami H, Murakami T, Terahara N, Mori M, Tsukui A. Induction of apoptosis in cultured human stomach cancer cells by potato anthocyanins and its inhibitory effects on growth of stomach cancer in mice. Food Sci Technol Res. 2006; 12:22–26.

8. Matsui T, Ebuchi S, Kobayashi M, Fukui K, Sugita K, Terahara N, Matsumoto K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. J Agric Food Chem. 2002; 50:7244–7248.

9. Shindo M, Kasai T, Abe A, Kondo Y. Effects of dietary administration of plant-derived anthocyanin-rich colors to spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 2007; 53:90–93.

10. Lu LZ, Zhou YZ, Zhang YQ, Ma YL, Zhou LX, Li L, Zhou ZZ, He TZ. Anthocyanin extracts from purple sweet potato by means of microwave baking and acidified electrolysed water and their antioxidation in vitro. Int J Food Sci Technol. 2010; 45:1378–1385.

11. Francis FJ. Food colorants: anthocyanins. Crit Rev Food Sci Nutr. 1989; 28:273–314.

12. Pensado L, Casais C, Mejuto C, Cela R. Optimization of the extraction of polycyclic aromatic hydrocarbons from wood samples by the use of microwave energy. J Chromatogr A. 2000; 869:505–513.

13. Sanoner P, Guyot S, Marnet N, Molle D, Drilleau JP. Polyphenol profiles of French cider apple varieties (Malus domestica sp.). J Agric Food Chem. 1999; 47:4847–4853.

14. Montilla EC, Hillebrand S, Butschbach D, Baldermann S, Watanabe N, Winterhalter P. Preparative isolation of anthocyanins from Japanese purple sweet potato (Ipomoea batatas L.) varieties by high-speed countercurrent chromatography. J Agric Food Chem. 2010; 58:9899–9904.

15. Sasaki Y, Ohba R. Antioxidant activity and optimal manufacturing conditions of purple sweet potato lactic acid bacteria drink. Food Sci Technol Res. 2004; 10:447–452.

16. Teow CC, Truong VD, McFeeters RF, Thompson RL, Pecota KV, Yencho GC. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007; 103:829–838.

17. Cho J, Kang JS, Long PH, Jing J, Back Y, Chung KS. Antioxidant and memory enhancing effects of purple sweet potato anthocyanin and cordyceps mushroom extract. Arch Pharm Res. 2003; 26:821–825.

18. Ye J, Meng X, Yan C, Wang C. Effect of purple sweet potato anthocyanins on beta-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochem Res. 2010; 35:357–365.

19. Liu S, Luo X, Li D, Zhang J, Qiu D, Liu W, She L, Yang Z. Tumor inhibition and improved immunity in mice treated with flavone from Cirsium japonicum DC. Int Immunopharmacol. 2006; 6:1387–1393.

20. Suda I, Oki T, Masuda M, Nishiba Y, Furuta S, Matsugano K, Sugita K, Terahara N. Direct absorption of acylated anthocyanin in purple-fleshed sweet potato into rats. J Agric Food Chem. 2002; 50:1672–1676.

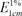 is the color value for the 1% extract in the 1-cm colorimetric cell at the maximum absorption wavelength, A is the absorbance, and m is the amount (0.2-0.7 g) of sample analyzed.
is the color value for the 1% extract in the 1-cm colorimetric cell at the maximum absorption wavelength, A is the absorbance, and m is the amount (0.2-0.7 g) of sample analyzed.



 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download