Abstract
BACKGROUND/OBJECTIVES
Sageretia thea is traditionally used as a medicinal herb to treat various diseases, including skin disorders, in China and Korea. This study evaluated the inhibitory effect of Sageretia thea fruit on melanogenesis and its underlying mechanisms in B16F10 mouse melanoma cells. The active chemical compounds in anti-melanogenesis were determined in
Sageretia thea.
MATERIALS/METHODS
Solvent fractions from the crude extract were investigated for anti-melanogenic activities. These activities and the mechanism of anti-melanogenesis in B16F10 cells were examined by determining melanin content and tyrosinase activity, and by performing western blotting.
RESULTS
The n-hexane fraction of Sageretia thea fruit (HFSF) exhibited significant anti-melanogenic activity among the various solvent fractions without reducing viability of B16F10 cells. The HFSF suppressed the expression of tyrosinase and tyrosinase-related protein 1 (TRP1). The reduction of microphthalmia-associated transcription factor (MITF) expression by the HFSF was mediated by the Akt/glycogen synthase kinase 3 beta (GSK3β) signaling pathway, which promotes the reduction of β-catenin. Treatment with the GSK3β inhibitor 6-bromoindirubin-3'-oxime (BIO) restored HFSF-induced inhibition of MITF expression. The HFSF bioactive constituents responsible for anti-melanogenic activity were identified by bioassay-guided fractionation and gas chromatography-mass spectrometry analysis as methyl linoleate and methyl linolenate.
The color of skin and hair results from the synthesis of melanin in melanocytes [1]. Melanin is generated by ultraviolet (UV) light and acts as a direct defense against UV radiation [2]. However, excessive melanin synthesis causes hyperpigmentation disorders, including melasma, freckles, lentigo, and other hyperpigmentation syndromes [3]. Melanosomes are specialized lysosome-related organelles that synthesize and store melanin pigments. Mature melanosomes are transferred from melanocytes to keratinocytes, leading to movement to the skin surface [4]. Several well-known enzymes, such as tyrosinase, tyrosinase-related protein (TRP) 1, and TRP2, play vital roles in melanosome biogenesis, and these proteins catalyze melanin synthesis [45]. Mutations in tyrosinase and TRP1 are involved in human pigment disorders, such as oculocutaneous albinism (OCA) 1 and OCA3, respectively [6].
Microphthalmia-associated transcription factor (MITF) is the core transcription factor involved in the expression and transport of melanosome component proteins [7]. The pivotal role of MITF is to control expression of melanogenic enzyme proteins including tyrosinase, TRP1, and TRP2, in addition to its role in the transport of melanosomes to the dendritic tips [38]. α-Melanocyte stimulating hormone (α-MSH) binds to melanocortin-1 receptor 1 (MC1R) and sequentially stimulates MITF expression [9]. MC1R triggers the cAMP pathway when activated, leading to cAMP-dependent transcriptional activation of MITF expression [10]. Although the role of glycogen synthase kinase 3 beta (GSK3β) in the induction of melanogenesis remains controversial, recent studies have reported that the inhibition of GSK3β phosphorylation induces the degradation of β-catenin and inhibits the transcriptional and protein expression of MITF and melanogenic enzyme proteins [11121314]. Melanogenesis stimulated by α-MSH increases the phosphorylation of GSK3β, leading to phosphorylation at Ser675 and stabilization of β-catenin protein. Subsequently, β-catenin accumulates in the cytoplasm by escaping ubiquitination-dependent proteasomal degradation, but relocalizes to the nucleus where it works in concert with cAMP response element binding protein (CREB) on the MITF promoter [15]. Therefore, exposure to GSK3β-specific inhibitors results in the induction of melanin synthesis and expression of tyrosinase and MITF [13].
Various skin depigmentation agents, such as arbutin, kojic acid, and hydroquinone, reduce melanin production [16]. However, these agents induce toxic, potentially carcinogenic side effects, and skin irritation [17181920]. Thus, whitening agents from natural sources are preferred and tend to dominate the cosmetic market [21]. Sageretia thea, belonging to the Rhamnaceae family, has long been recognized as a medicinal herb in Korea and China [22232425]. Several studies have demonstrated that the leaves of Sageretia thea have antioxidant activity [2223] and have been used to treat itching, boils, and inflammation of the skin caused by lacquer poison in China [25]. Although some studies have demonstrated the biological functions of Sageretia thea leaves, few studies have investigated the chemical and biological composition of Sageretia thea fruits. Notably, the fruit constitutes an important source of active metabolites against skin aging; thus, several studies have reported on melanogenic-inhibitory activities and fruit constituents [262728]
In the present study, we examined the anti-melanogenesis effect of Sageretia thea fruit and its underlying molecular mechanisms in B16F10 cells in an effort to develop new, safe, and effective skin lighteners from natural sources. Various solvent fractions from the crude extract were examined to obtain fractions with greater anti-melanogenic effects, and a gas chromatograpy-mass spectroscopy (GC-MS) analysis was conducted to identify the potential ingredients from the active solvent fractions. We identified the major anti-melanogenic compounds (methyl linoleate and methyl linolenate) from the n-hexane fraction, and we measured melanin content and intracellular tyrosinase inhibitory activity in B16F10 cells. Furthermore, we determined the levels of tyrosinase, TRP1, MITF, Akt/p-Akt, GSK3β/p-GSK3β, and β-catenin by western blotting and elucidated the biological mechanisms underlying the inhibitory effect of melanogenesis in the n-hexane fraction from Sageretia thea fruit (HFSF), as well as those of methyl linoleate and methyl linolenate.
Methyl linoleate, methyl linolenate, α-MSH, L-DOPA, sodium hydroxide (NaOH), mushroom tyrosinase, arbutin, kojic acid, resveratrol, and 6-bromoindirubin-3'-oxime (BIO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies recognizing Akt, p-Akt, GSK3β, p-GSK3β, and β-actin were obtained from Cell Signaling Technology (Beverly, MA, USA). Anti-β-catenin antibody was purchased from BD (Franklin Lakes, NJ, USA), and anti-MITF, anti-tyrosinase, and anti-TRP1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Sageretia thea fruit was harvested from Seogwang, Jeju Island, South Korea in May 2014. Botanical samples were identified taxonomically by Dr. Hui Kim, Mokpo National University, Korea. Sageretia thea fruit (10 kg) was extracted with 80% aqueous methanol for 24 h. After concentrating the methanol extract, the crude extract (1.6 kg) was partitioned with n-hexane, chloroform, ethyl acetate, n-butanol, and water. The fractions were generated using 18 g of n-hexane, 3.6 g of chloroform, 20.9 g of ethyl acetate, 135.0 g of n-butanol, and 1,422.5 g of water. The n-hexane fraction was further partitioned using a silica gel column and Sephadex LH-20 column. In total, 18 fractions (H-1-H-18) were obtained and monitored by thin-layer chromatography. After fraction H-3 (1.47 g) was partitioned using a silica gel column, nine fractions (H-3-1-H-3-9) were obtained and monitored by TLC.
GC-MS analysis was performed with a Shimadzu model QP-2010 (Kyoto, Japan) in EI mode (70 eV) using a capillary Rtx-5MS column (30 m × 0.25 mm, 0.25 µm film thickness). A 1 µL volume was injected with a 1:10 split ratio at temperatures of 250℃ at the inlet and 290℃ at the interface. An initial temperature of 60℃ for 2 min was increased to 250℃ at a rate of 5℃/min and then increased to 310℃ at a rate of at 8℃/min for 12 min. Mass range was scanned from 40 to 500 amu. Data were identified using the WILEY9 and NIST05 libraries.
B16F10 mouse melanoma and human dermal fibroblast cells were kindly provided by Dr. Nam Ho Lee and Dr. Moonjae Cho, Jeju National University, Korea, respectively. The cells were cultured in DMEM with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen, Grand Island, NY, USA) in a 5% CO2 atmosphere at 37℃. Cell viability was examined using the MTT assay (Amresco Inc., Solon, OH, USA). Briefly, cells were treated with various concentrations of samples. After 48 h, the MTT solution was added to the cells for 4 h. After solubilizing the formazan crystals with DMSO, the absorbance at 570 nm was detected using a microplate reader (Tecan, Salzburg, Austria).
Using a slightly modified version of the method described previously, we conducted an assay to determine the melanin content [29]. Cells were treated with various concentrations of HFSF in the absence or presence of α-MSH for 48 h. After harvesting the cells by centrifugation, the pellets were dissolved with 1 N NaOH and 10% DMSO at 80℃ for 30 min, and the absorbance was measured at 475 nm using the microplate reader.
Intracellular tyrosinase activity assays were performed according to a slightly modified version of the method described previously [16]. Cells were treated with various concentrations of HFSF in the absence or presence of α-MSH for 48 h. The cells were homogenized with cold RIPA buffer and protease inhibitor cocktail. The protein content was normalized with a BSA protein assay kit (Pierce, Rockford, IL, USA) and then incubated with L-DOPA (2 mM) at 37℃ for 30 min. Direct tyrosinase activity was determined using a mushroom tyrosinase solution. Samples of varying dilutions were incubated with L-DOPA (2 mM) at 37℃ for 20 min. Tyrosinase activity was detected using a microplate reader at an absorbance of 490 nm. in situ tyrosinase activity was assessed as described previously [30]. Cells were treated with various samples in the absence or presence of α-MSH for 48 h. The cells were fixed with 4% paraformaldehyde for 40 min. After permeabilizing the cell with 0.1% Triton X-100, cells were incubated in L-DOPA (2 mM) for 2 h at 37℃. The cells were examined using a microscope (Olympus, Essex, UK).
Cells were treated with various concentrations of HFSF in the absence or presence of α-MSH for 48 h. The cells were homogenized with cold RIPA buffer with protease inhibitor cocktail and then normalized using a BSA protein assay kit. The cell lysates were separated by 12% SDS-PAGE and then transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 5% nonfat dried milk. After incubating the membrane with primary antibodies, the membrane was probed using horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG secondary antibodies for 30 min. Specific proteins were detected using the BS ECL Plus kit (Biosesang, Gyeonggi-do, Korea).
All data analysis was performed by one-way analysis of variance (ANOVA) using SPSS (SPSS Inc., Chicago, IL, USA). The data were analyzed as means ± standard deviation (SD). At least three independent experiments were conducted for each experiment. P values < 0.05 or < 0.01 were considered to indicate statistical significance.
The Sageretia thea fruit crude 80% methanol extract and its n-hexane, chloroform, ethyl acetate, n-butanol, and water fractions at concentrations of 25 and 50 µg/mL were compared for their inhibitory activities against melanin production in α-MSH-induced B16F10 cells. The n-hexane fraction from Sageretia thea fruit (HFSF) with the highest inhibitory activity against melanin production was chosen among these fractions (Fig. 1A). As shown in Fig. 1B, no obvious toxicity in B16F10 melanoma cells was observed when the HFSF concentration reached 100 µg/mL. The HFSF affected growth of human dermal primary fibroblast cells when its concentration was ≥ 100 µg/mL (Fig. S1A). Thus, ≤ 100 µg/mL of the HFSF was used in subsequent experiments. This non-toxic HFSF treatment significantly suppressed melanin production in a concentration-dependent manner (Fig. 1C). Moreover, the anti-melanogenic effec of HFSF was superior to those of three common whitening agents named arbutin (2 mM), kojic acid (400 µM), and resveratrol (20 µM).
Tyrosinase induces the oxidation of tyrosine as a first step of melanogenesis [31]. Therefore, we treated B16F10 cells with the HFSF to examine the tyrosinase activity. α-MSH-stimulated tyrosinase activity was reduced in a concentration-dependent manner by the HFSF (Fig. 2A). In addition, when B16F10 cells were incubated with L-DOPA to detect in situ tyrosinase activity, we observed that HFSF reduced the staining in cells in the presence of L-DOPA (Fig. 2B). In contrast, no difference in mushroom tyrosinase activity was observed between treatment with or without the HFSF (Fig. S1B). These results indicate that cellular processes were modulated to decrease tyrosinase activity. Next, we examined the expression of melanogenic-related enzyme proteins by western blotting. As shown in Fig. 2C, treatment with the HFSF significantly diminished the levels of tyrosinase and TRP1 in a concentration-dependent manner. However, no change in TRP2 protein expression was detected (data not shown). These results indicated that suppression of melanogenesis with the HFSF was related to interrupting the upstream pathway that regulates the expression of tyrosinase and TRP1.
We determined the effect of the HFSF on MITF and phosphorylation of GSK3β to elucidate the mechanism by which it inhibited melanin synthesis. The GSK3β/β-catenin signal pathway is closely related to melanin synthesis [32]. Inhibition of GSK3β and concurrent accumulation of β-catenin result in activation of the MITF promoter during α-MSH-stimulated melanogenesis and ultimately MITF target genes, such as tyrosinase, TRP1, and TRP2 [333435]. Previous reports suggested that activated Akt phosphorylates GSK3β and inhibits degradation of β-catenin [1433]. As shown in Fig. 2D, α-MSH treatment significantly increased the MITF level, whereas co-treatment with HFSF had a concentration-dependent inhibitory effect on the MITF level. The data show that the level of GSK3β phosphorylation decreased, while the total GSK3β protein expression level remained unchanged. As expected, the expressions of phosphorylated Akt and β-catenin were decreased significantly in B16F10 cells treated with the HFSF (Fig. 2D). To confirm whether the expression of the β-catenin protein is directly regulated by GSK3β in HFSF-treated cells, the cells were treated with the GSK3β inhibitor BIO. The expression levels of MITF and β-catenin increased in response to co-treatment with 2 µM BIO compared to those of 100 µg/mL HFSF alone (Fig. 2E). These results indicated that the HFSF suppresses the expression of MITF via the Akt/GSK3β/β-catenin signal pathway, thereby diminishing the expression of melanogenic enzymes and ultimately reducing melanin production.
As the HFSF inhibited melanogenesis, we conducted bioassay-guided fractionation of the HFSF. The HFSF was further separated by silica gel column chromatography into 18 sub-fractions (H-1-H-18), using methanol as the mobile phase. The cells treated with sub-fraction H-3 inhibited melanin production the most among all of the sub-fractions (Table S1). H-3 was subjected to further fractionation into nine sub-fractions (H-3-1-H-3-9), which were evaluated for their inhibitory activity against melanin production. H-3-1 was the sub-fraction that inhibited melanin content most in α-MSH-stimulated B16F10 cells (Table S2). To compare the melanin content, H-3 and H-3-1 were tested at the same concentrations as the HFSF; the inhibitory activities of melanin production were enriched in sub-fraction H-3-1 (Fig. 3A). Moreover, these fractions inhibited in situ tyrosinase activity in a manner similar to that observed with the melanin content (Fig. S2). Next, we analyzed the chemical profiles of the HFSF, H-3, and H-3-1 to identify the constituents responsible for anti-melanogenesis. GC-MS analysis revealed that methyl linoleate (21.03 ± 6.73%) and methyl linolenate (20.97 ± 6.68%) were the major constituents of the HFSF, whereas methyl linoleate, methyl linolenate, and β-sitosterol were major constituents of H-3 with contents of 21.99 ± 0.97%, 23.77 ± 1.61, and 20.57 ± 2.24%, respectively (Table 1). Similarly, the major constituents of H-3-1 were methyl linoleate (39.24 ± 1.28%) and methyl linolenate (41.57 ± 5.25%), which were found in the HFSF, H-3, and H-3-1 and were enriched in sub-fraction H-3-1 (Fig. 3B–D). These results reveal that the anti-melanogenic activity of the HFSF was likely due to the presence of methyl linoleate and methyl linolenate.
As methyl linoleate and methyl linolenate were major constituents of H-3-1, we examined the anti-melanogenic effect of methyl linoleate and methyl linolenate in α-MSH-stimulated B16F10 cells. Cell viability was not affected at concentrations ≤ 100 µM methyl linoleate or methyl linolenate. However, cell viability decreased to 73.70 ± 5.57% in response to 200 µM methyl linolenate, but not to 200 µM methyl linoleate (Fig. 4A and B). Furthermore, neither compound was toxic to human dermal primary fibroblast cells at 200 µM (Fig. S3A and B). Therefore, 100 µM, which showed no toxicity to either cell type, was chosen to determine the anti-melanogenic effects of methyl linoleate and methyl linolenate in B16F10 cells. As shown in Fig. 4C, α-MSH-stimulated melanin production was almost completely blocked by methyl linoleate and methyl linolenate. Similarly, methyl linoleate and methyl linolenate decreased α-MSH-stimulated intracellular tyrosinase activity and in situ tyrosinase activity in concentration-dependent manners (Fig. 4D and E). Moreover, the inhibitory activities of methyl linolenate on melanin production and intracellular tyrosinase activities were stronger than those of methyl linoleate. Notably, treatment with methyl linoleate and methyl linolenate showed no inhibitory effect on mushroom tyrosinase activity (Fig. S3C). As shown in Fig. 5A, methyl linoleate and methyl linolenate inhibited the expression of tyrosinase and TRP1 in α-MSH-treated B16F10 cells, suggesting that methyl linoleate and methyl linolenate inhibited α-MSH-induced intracellular tyrosinase activity by lowering expression of the melanogenic enzymes. Consistent with the HFSF treatment, methyl linoleate and methyl linolenate decreased MITF expression and Akt and GSK3 β phosphorylation, respectively (Fig. 5B). Moreover, decrease in the β-catenin level was detected in B16F10 cells treated with methyl linoleate and methyl linolenate. These results suggest that methyl linoleate and methyl linolenate suppressed melanin production by suppressing MITF in an Akt/GSK3β/β-catenin-dependent manner, resulting in decreased tyrosinase and TRP1 expression. Therefore, the anti-melanogenic effect of the HFSF was attributed to methyl linoleate and methyl linolenate. Taken together, our results suggest that suppressing MITF through the Akt/GSK3β/β-catenin pathway mediated the anti-melanogenic effect of the HFSF, which contains methyl linoleate and methyl linolenate as major constituents.
The fruits of Sageretia thea are purple and contain micronutrients [24]. Sageretia thea fruits contain a much higher level of anthocyanins than do blueberries [2436]. Anthocyanins are beneficial bioactive flavonoids known to reduce melanin production and mushroom tyrosinase inhibitory activity in α-MSH-stimulated B16F10 cells [37]. Therefore, we expected that Sageretia thea fruits would have a whitening effect derived from the presence of a large number of anthocyanins. However, we found little anthocyanin present in the n-hexane and ethyl acetate fractions of Sageretia thea fruits. Notably, in this study, bioassay-guided fractionation of the HFSF followed by GC-MS analysis identified methyl linoleate and methyl linolenate as major constituents. Interestingly, methyl linoleate and methyl linolenate isolated from Oxalis triangularis significantly block forskolin-induced melanogenesis by inhibiting cAMP production and tyrosinase promoter activity in B16 mouse melanoma cells [38]. However, no analysis of melanogenic protein expression levels was conducted in that study. In agreement with this previous report, we observed that treatment with methyl linoleate and methyl linolenate decreased melanin content. Furthermore, our data showed that methyl linoleate and methyl linolenate decreased the expression of MITF, tyrosinase, and the TRP1 protein and reduced intracellular tyrosinase activity in α-MSH-treated B16F10 cells, suggesting that the anti-melanogenic activities of methyl linoleate and methyl linolenate are attributed to inhibited transcriptional activation of MITF expression.
In the process of melanin synthesis, tyrosinase initiates melanogenesis via the oxidation of tyrosine and then yields L-DOPA and dopaquinone as the final common precursors [33]. Tyrosinase plays a role in regulating general melanin synthesis as a first and rate-limiting enzyme [31]. Additionally, melanin synthesis requires TRP1 and TRP2, which catalyze specific steps in melanogenesis [39]. Moreover, a strong intermolecular association between TRP1 and tyrosinase has been demonstrated to stabilize tyrosinase [40]. Our data indicated that the intracellular tyrosinase inhibitory activity of the HFSF was similar to that of resveratrol, a tyrosinase inhibitor positive control. When cells were treated with 100 µg/mL HFSF, the intracellular tyrosinase inhibitory activity of HFSF was similar to that of 20 µM of resveratrol, a positive control of tyrosinase inhibitor, as shown in Fig. 2A. Interestingly, the reduction of TRR1 expression with 100 µg/mL of HFSF was more pronounced than with 20 µM resveratrol, affecting stabilization and tyrosinase activity (Fig. 2C). Additionally, the HFSF contained γ-tocopherol (0.72 ± 0.15%) and ethyl linoleate (2.02 ± 0.19%), which have inhibitory effects on melanogenesis (Table 1) [3841]. Thus, the anti-melanogenic effect of the HFSF could be derived from the synergistic action of γ-tocopherol and ethyl linoleate, as well as methyl linoleate and methyl linolenate. Therefore, the HFSF itself could have potential as a whitening agent.
A relationship between Wnt/β-catenin signaling and MITF was demonstrated previously by physical interactions between the β-catenin protein and T-cell transcription factor/lymphoid enhancer binding factor, which directly binds and regulates the MITF gene to activate MITF target genes encoding tyrosinase, TRP1, and TRP2 [34]. Notably, α-MSH stimulates phosphorylation of GSK3β at Ser9 in a protein kinase A-dependent fashion resulting in inactivation of GSK3β. The attenuation of GSK3β activity facilitates stabilization of β-catenin [15]. α-MSH stimulation also stabilizes β-catenin by a dual mechanism in which p21-activated kinase 4 (PAK) induces the phosphorylation of β-catenin at Ser675 while blocking β-catenin phosphorylation at Ser33/37 [42]. The accumulated β-catenin in the cytoplasm is translocated into the nucleus where it combines with the MITF promoter contributing to transcription of MITF [15]. In addition, activation of Akt phosphorylates GSK3β at Ser9, which eventually inhibits degradation of β-catenin [1443]. Our data indicate that the HFSF attenuated phosphorylation of Akt and GSK3β, in other words, the inactive form of GSK3β decreased, leading to a reduction in β-catenin, and a decrease in MITF expression. Treatment with BIO, a kind of GSK3β inhibitor, restored HFSF-induced β-catenin inhibition. Similarly, we observed that inhibiting MITF with methyl linoleate and methyl linolenate was dependent on the Akt/GSK3/β-catenin pathway, and treatment with BIO reversed the reduction in β-catenin expression caused by methyl linoleate and methyl linolenate (data not shown). Therefore, the HFSF, methyl linoleate, and methyl linolenate inhibit β-catenin-mediated transcriptional activation of MITF through the Akt/GSK3β signaling pathway.
In conclusion, this study is the first to demonstrate that Sageretia thea fruit possesses anti-melanogenic activity without being toxic to B16F10 cells. The HFSF suppressed melanin content and intracellular tyrosinase activity by downregulating the expression of tyrosinase, TRP1, and MITF. Notably, the methyl linoleate and methyl linolenate were identified as major constituents of HFSF. We demonstrated that the underlying mechanism of anti-melanogenic activity of the HFSF, methyl linoleate, and methyl linolenate was the inhibition of MITF expression mediated by the Akt/GSK3β/β-catenin pathway. Hence, HFSF, methyl linoleate and methyl linolenate could be applied as whitening agents in cosmetics and in the clinic for treating hyperpigmentation disorders.
Figures and Tables
Fig. 1
Effects of the n-hexane fraction of Sageretia thea fruit (HFSF) on cell viability and melanin content in B16F10 cells.
(A) Melanin content was examined after treatments with 25 and 50 µg/mL of the Sageretia thea fruit crude extract and its fractions or 20 µM of resveratrol. The melanin levels visualized after treatment with the Sageretia thea fruit methanol extract and its fractions at concentration of 50 µg/mL. Cells stimulated with α-melanocyte stimulating hormone (α-MSH) for 48 h. (B) Cell toxicity of the HFSF was evaluated by MTT assay. (C) Melanin content was visualized and examined after treatments with the indicated concentrations of the HFSF or known whitening agents (AR, arbutin 2 mM; KA, kojic acid 400 µM; RSV, resveratrol 20 µM). Cells were stimulated with α-MSH for 48 h. Data are represented as means ± SD. *P < 0.05, **P < 0.01 versus α-MSH (500 nM) control; #P < 0.01 versus control. ME, methanol extract of Sageretia thea fruit; HF, n-hexane fraction from ME (HFSF); CF, chloroform fraction from ME; EF, ethyl acetate fraction from ME; BF, n-butanol fraction from ME; WF, water fraction from ME.
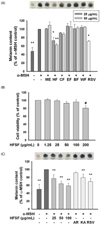
Fig. 2
The n-hexane fraction of Sageretia thea fruit (HFSF) suppresses tyrosinase activity and expression by decreasing MITF expression.
B16F10 cells were treated with the HFSF or resveratrol (RSV, 20 µM) in medium containing α-MSH. (A) Intracellular tyrosinase activity and (B) in situ tyrosinase activity were determined after a 48 h treatment with the HFSF or resveratrol. Images were captured using microscopy Bar = 20 µm. (C) The expression of tyrosinase and tyrosinase-related protein 1 (TRP1) protein was detected by western blotting after the HFSF treatment for 48 h. (D) Protein expression levels, including MITF, Akt, p-Akt, GSK3β, p-GSK3β, and β-catenin were examined by western blotting. Cells were treated with the HFSF for 4 h. (E) The effects of the HFSF (100 µg/mL) on β-catenin expression were examined by western blot in the absence or presence of 6-bromoindirubin-3'-oxime (BIO, 2 µM) for 4 h. Data are represented as means ± SD.
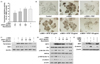
Fig. 3
Effects of sub-fractions from the n-hexane fraction of Sageretia thea fruit (HFSF) on anti-melanogenesis in B16F10 cells.
(A) Melanin content was examined after treatment with the HFSF, sub-fraction H-3 from the HFSF (H-3), sub-fraction H-3-1 from H-3 (H-3-1), or resveratrol (RSV, 20 µM) in medium containing α-MSH for 48 h. Melanin levels were visualized in the 50 µg/mL treatment concentration. Representative GC-MS chromatogram of HFSF (B), H-3 (C), and H-3-1 (D). Assigned peak numbers correspond to the number of identified compounds in Table 1. (1) methyl palmitate, (3) methyl linoleate, (4) methyl linolenate, (6) methyl stearate. Data are represented as means ± SD. *P < 0.05, **P < 0.01 versus α-MSH (500 nM) control.
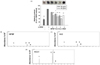
Fig. 4
Effects of methyl linoleate (ML) and methyl linolenate (MLN) on melanogenesis in B16F10 cells.
Cell toxicity of methyl linoleate (A) and methyl linolenate (B) was evaluated by the MTT assay. (C) Melanin content was visualized and determined after the ML, MLN, or positive whitening agent treatments (AR, arbutin 2 mM; KA, kojic acid 400 µM; RSV, resveratrol 20 µM) in α-MSH-supplemented medium for 48 h. Intracellular tyrosinase activity (D) and in situ tyrosinase activity (E) were determined after the ML, MLN, or resveratrol treatments in α-MSH-supplemented medium for 48 h. Images were captured using microscopy. Bar = 20 µm. Data are represented as means ± SD. **P < 0.01 versus α-MSH (500 nM) control; #P < 0.01 versus control.
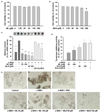
Fig. 5
Effects of methyl linoleate (ML) and methyl linolenate (MLN) on melanogenic-related proteins in B16F10 cells.
(A) Cells were treated with ML or MLN (50 µM, 100 µM) in the absence or presence of α-MSH (500 nM) for 48 h. Western blotting was conducted to examine the expression levels of tyrosinase and TRP1 protein. (B) Cells were treated with ML or MLN (100 µM) in the absence or presence of α-MSH for 4 h. The expression levels of protein including MITF, Akt, p-Akt, GSK3β, p-GSK3β, and β-catenin were detected by western blot. The bar graphs represent the band intensity compared to the α-MSH control. Data are represented as means ± SD.
Notes
References
1. Swope VB, Jameson JA, McFarland KL, Supp DM, Miller WE, McGraw DW, Patel MA, Nix MA, Millhauser GL, Babcock GF, Abdel-Malek ZA. Defining MC1R regulation in human melanocytes by its agonist alpha-melanocortin and antagonists agouti signaling protein and beta-defensin 3. J Invest Dermatol. 2012; 132:2255–2262.

2. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007; 128:853–864.

3. Speeckaert R, Van Gele M, Speeckaert MM, Lambert J, van Geel N. The biology of hyperpigmentation syndromes. Pigment Cell Melanoma Res. 2014; 27:512–524.

4. Yatsu A, Ohbayashi N, Tamura K, Fukuda M. Syntaxin-3 is required for melanosomal localization of Tyrp1 in melanocytes. J Invest Dermatol. 2013; 133:2237–2246.

5. Zhang P, Liu W, Zhu C, Yuan X, Li D, Gu W, Ma H, Xie X, Gao T. Silencing of GPNMB by siRNA inhibits the formation of melanosomes in melanocytes in a MITF-independent fashion. PLoS One. 2012; 7:e42955.

6. Zhang Y, Helke KL, Coelho SG, Valencia JC, Hearing VJ, Sun S, Liu B, Li Z. Essential role of the molecular chaperone gp96 in regulating melanogenesis. Pigment Cell Melanoma Res. 2014; 27:82–89.

7. Ho H, Ganesan AK. The pleiotropic roles of autophagy regulators in melanogenesis. Pigment Cell Melanoma Res. 2011; 24:595–604.

8. Kim ES, Park SJ, Goh MJ, Na YJ, Jo DS, Jo YK, Shin JH, Choi ES, Lee HK, Kim JY, Jeon HB, Kim JC, Cho DH. Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of MITF via ROS-ERK activation. Pigment Cell Melanoma Res. 2014; 27:1051–1062.

9. Beaumont KA, Smit DJ, Liu YY, Chai E, Patel MP, Millhauser GL, Smith JJ, Alewood PF, Sturm RA. Melanocortin-1 receptor-mediated signalling pathways activated by NDP-MSH and HBD3 ligands. Pigment Cell Melanoma Res. 2012; 25:370–374.

10. Abrisqueta M, Herraiz C, Pérez Oliva AB, Sanchez-Laorden BL, Olivares C, Jiménez-Cervantes C, García-Borrón JC. Differential and competitive regulation of human melanocortin 1 receptor signaling by beta-arrestin isoforms. J Cell Sci. 2013; 126:3724–3737.
11. Khaled M, Larribere L, Bille K, Aberdam E, Ortonne JP, Ballotti R, Bertolotto C. Glycogen synthase kinase 3β is activated by cAMP and plays an active role in the regulation of melanogenesis. J Biol Chem. 2002; 277:33690–33697.

12. Takeda K, Takemoto C, Kobayashi I, Watanabe A, Nobukuni Y, Fisher DE, Tachibana M. Ser298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum Mol Genet. 2000; 9:125–132.

13. Bellei B, Flori E, Izzo E, Maresca V, Picardo M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal. 2008; 20:1750–1761.

14. Zhu PY, Yin WH, Wang MR, Dang YY, Ye XY. Andrographolide suppresses melanin synthesis through Akt/GSK3beta/beta-catenin signal pathway. J Dermatol Sci. 2015; 79:74–83.

15. Bellei B, Pitisci A, Catricalà C, Larue L, Picardo M. Wnt/beta-catenin signaling is stimulated by alpha-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation. Pigment Cell Melanoma Res. 2011; 24:309–325.

16. Hwang E, Lee TH, Lee WJ, Shim WS, Yeo EJ, Kim S, Kim SY. A novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+ influx via TRPM1 channels. Pigment Cell Melanoma Res. 2016; 29:81–91.

17. Chung KW, Jeong HO, Jang EJ, Choi YJ, Kim DH, Kim SR, Lee KJ, Lee HJ, Chun P, Byun Y, Moon HR, Chung HY. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim Biophys Acta. 2013; 1830:4752–4761.

18. García-Gavín J, González-Vilas D, Fernández-Redondo V, Toribio J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermatitis. 2010; 62:63–64.

19. Takizawa T, Imai T, Onose J, Ueda M, Tamura T, Mitsumori K, Izumi K, Hirose M. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl) nitrosamine or N-diethylnitrosamine. Toxicol Sci. 2004; 81:43–49.

20. Hong YH, Jung EY, Noh DO, Suh HJ. Physiological effects of formulation containing tannase-converted green tea extract on skin care: physical stability, collagenase, elastase, and tyrosinase activities. Integr Med Res. 2014; 3:25–33.

21. Chiang HM, Chien YC, Wu CH, Kuo YH, Wu WC, Pan YY, Su YH, Wen KC. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem Toxicol. 2014; 65:129–139.

22. Chung SK, Kim YC, Takaya Y, Terashima K, Niwa M. Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. J Agric Food Chem. 2004; 52:4664–4668.

23. Chung SK, Chen CY, Blumberg JB. Flavonoid-rich fraction from Sageretia theezans leaves scavenges reactive oxygen radical species and increases the resistance of low-density lipoprotein to oxidation. J Med Food. 2009; 12:1310–1315.

24. Hyun TK, Song SC, Song CK, Kim JS. Nutritional and nutraceutical characteristics of Sageretia theezans fruit. J Food Drug Anal. 2015; 23:742–749.

25. Song SC. Characteristics of Sageretia thea (Osbeck) M.C. Johnst native to Jeju Island and effects of plant growth regulator treatments on fruit quality [Ph.D. thesis]. Jeju: Jeju National University;2014.
26. Akihisa T, Tochizawa S, Takahashi N, Yamamoto A, Zhang J, Kikuchi T, Fukatsu M, Tokuda H, Suzuki N. Melanogenesis-inhibitory saccharide fatty acid esters and other constituents of the fruits of Morinda citrifolia (noni). Chem Biodivers. 2012; 9:1172–1187.

27. Diwakar G, Rana J, Saito L, Vredeveld D, Zemaitis D, Scholten J. Inhibitory effect of a novel combination of Salvia hispanica (chia) seed and Punica granatum (pomegranate) fruit extracts on melanin production. Fitoterapia. 2014; 97:164–171.

28. Nam JH, Lee DU. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J Dermatol Sci. 2016; 84:305–313.

29. Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985; 45:1474–1478.
30. Newton RA, Cook AL, Roberts DW, Leonard JH, Sturm RA. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol. 2007; 127:2216–2227.

31. Lee EJ, Lee YS, Hwang S, Kim S, Hwang JS, Kim TY. N-(3,5-dimethylphenyl)-3-methoxybenzamide (A(3)B(5)) targets TRP-2 and inhibits melanogenesis and melanoma growth. J Invest Dermatol. 2011; 131:1701–1709.

32. Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000; 275:14013–14016.

33. Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch Biochem Biophys. 2014; 563:28–34.

34. Hwang I, Park JH, Park HS, Choi KA, Seol KC, Oh SI, Kang S, Hong S. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J Dermatol Sci. 2013; 72:274–283.

35. Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998; 8:573–581.

36. Routray W, Orsat V. Blueberries and their anthocyanins: factors affecting biosynthesis and properties. Compr Rev Food Sci Food Saf. 2011; 10:303–320.

37. Aramwit P, Bang N, Srichana T. The properties and stability of anthocyanins in mulberry fruits. Food Res Int. 2010; 43:1093–1097.

38. Huh S, Kim YS, Jung E, Lim J, Jung KS, Kim MO, Lee J, Park D. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol Pharm Bull. 2010; 33:1242–1245.

39. Jung H, Chung H, Chang SE, Choi S, Han IO, Kang DH, Oh ES. Syndecan-2 regulates melanin synthesis via protein kinase C betaII-mediated tyrosinase activation. Pigment Cell Melanoma Res. 2014; 27:387–397.

40. Jiménez-Cervantes C, Martínez-Esparza M, Pérez C, Daum N, Solano F, García-Borrón JC. Inhibition of melanogenesis in response to oxidative stress: transient downregulation of melanocyte differentiation markers and possible involvement of microphthalmia transcription factor. J Cell Sci. 2001; 114:2335–2344.

41. Kamei Y, Otsuka Y, Abe K. Comparison of the inhibitory effects of vitamin E analogues on melanogenesis in mouse B16 melanoma cells. Cytotechnology. 2009; 59:183–190.

Supplementary data
Table S1
Effects of the n-hexane fraction of Sageretia thea fruit (HFSF) sub-fractions on melanin content in B16F10 cells.
Fig. S1
Effects of the n-hexane fraction of Sageretia thea fruit (HFSF) on cell viability and mushroom tyrosinase activity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download