Abstract
Atherosclerotic renovascular hypertension is a form of secondary hypertension due to renal artery stenosis. After the introduction of medical therapy such as with statins and angiotensin blocking agents, it has been considered a very slowly progressive disease. In the 1990s, surgical methods were compared to radiological intervention and showed no additional benefits. Recent clinical data also demonstrate that in cases of relatively stable atherosclerotic renovascular disease, medical therapy is as effective as other interventions with regard to patient outcomes. In this paper the recent clinical outcomes are reviewed.
Atherosclerotic renal artery hypertension is reported in almost 7% of adults older than 65 years1) and is associated with cardiovascular events, and may double risk of mortality2). In the 1990s, radiological angioplasty began to replace surgical revascularization. Galaria et al. showed that percutaneous and open renal revascularization had equivalent long-term functional outcomes3). Over time, the less-invasive procedure became more accessible.
Most cases of atherosclerotic renovascular disease (ARVD) are located in the ostium, and are extensions of calcified aortic plaques4). These lesions tend to return to their original shape with balloon angioplasty alone. Stent placement might also provide additional force to increase the rates of technical success5) and to reduce the rate of restenosis at 6 months after the initial procedure6). Intervention with stents has become a standard procedure; in patients with stenosis of the renal artery, placement of a stent is likely to be the initial form of treatment. However, there is limited evidence to support revascularization over medical therapy for patients with atherosclerotic renal artery stenosis7, 8).
Retrospective studies previously reported that renal artery lesions might progress to severe stenosis and ultimately to renal artery occlusion9). Michael et al. performed a prospective study that reported that the progression of renal artery disease was a frequent occurrence with an annual rate of progression of renal artery lesions reported to be 7%10). However, at the time of these studies, statins were being used in fewer patients. A retrospective study of the effects of statins on the progression of ARVD has shown that the use of statins reduces the risk of progression and the development of ARVD11). Another prospective population-based study reported that the progression to significant ARVD was observed in only 4.0% during 8 years of follow-up (annualized rate, 0.5% per year), and no case of ARVD progressed to occlusion12).
Hypertension and renal artery stenosis are not necessarily renovascular hypertension. Essential hypertension and clinically silent renal artery stenosis often coexist, and essential hypertension also coexists with renovascular hypertension13). This is why blood pressure control does not always improve after stenting.
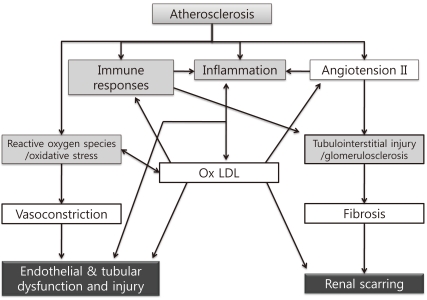
Renal injury distal to an atherosclerotic renovascular obstruction is due to multiple intrinsic factors producing parenchymal tissue injury (Fig. 1)14). Non-traditional mediators of ARVD such as inflammatory pathways, reactive oxygen species production, ischemia/reperfusion damage and modulation of matrix turnover have been proposed as causes of the renal failure related to ARVD15). This complexity of the pathophysiology might explain why the severity of the stenosis is not correlated with renal dysfunction.
Clinical data support dissociation of improved renal artery patency from clinical outcome in patients with atherosclerotic renal artery stenosis; this may illustrate the effects of irreversible injury on the post stenotic kidney. A prospective clinical study reported by Wright et al. showed absence of a correlation between renal artery anatomy and baseline renal function or functional outcome, and a good correlation between renal functional outcome and proteinuria; these findings suggest that renal parenchymal damage is a major determinant of renal dysfunction and outcome rather than the severity of the renal artery stenosis in ARVD16). Radermacher et al. reported that the mean arterial pressure did not decrease by 10 mmHg or more after revascularization and renal function declined in most patients with ARVD that had high resistance-index values before revascularization17). Chrysochou et al. showed a correlation between baseline proteinuria and decline in estimated glomerular filtration rate (GFR) with time after revascularization18). Therefore, post stenotic renal injury can lead to renal parenchymal injury reflected by high intrarenal resistance and/or the presence of proteinuria. Proteinuria and other factors involved in intrarenal resistance are predictors of a poor outcome after renal revascularization in ARVD.
When patients with chronic kidney disease undergo renal angioplasty with/without stent placement, the response with regard to renal function can be negative as well as positive. Positive responses include improvement of renal function, and stabilization or attenuation of declining renal function, as expected. However, sometimes declining renal function is accelerated after revascularization. Possible causes of these adverse outcomes include contrast induced nephropathy, atheroembolism, and restenosis or stent thrombosis19). The administration of contrast might increase the risk of acute renal dysfunction, especially in patients that have preexisting renal impairment. An ex vivo study reported that each manipulation of atheroma specimens, from simply advancing the guidewire through the atherosclerotic lesion to positioning and deploying the Wallstent, releases thousands of fragments, and that these atherosclerotic fragments are of sufficient size to create vascular occlusion and initiate significant renal parenchymal damage20). Currently, there is no way to predict positive or negative responses after revascularization.
ARVD is a cardiovascular condition that is associated with renal artery stenosis. The nature of this disorder suggests that a systemic approach is necessary to provide cardiovascular protection. Previously, statin therapy was discussed as a method of altering the natural history of ARVD11). Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers effectively reduce blood pressure in patients with renovascular disease. In addition, a population-based cohort study of over 3,500 patients with ARVD in Canada found that angiotensin inhibitors could cause acute renal toxicity in a small subset of vulnerable patients; however, they still improved the cardiovascular and renal outcomes in patients with ARVD, but at the expense of acute renal toxicity21).
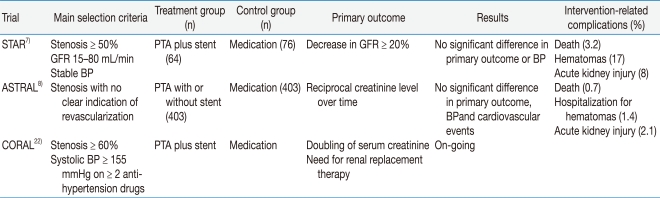
Recently three randomized controlled trials have been performed: the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial, the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial, and the Cardiovascular Outcomes in Renal Artherosclerotic Lesions (CORAL) trial (Table 1). Two large randomized trials of intervention vs. medical therapy showed negative results for the intervention. The last trial is under way. The STAR trial was a European multicenter trial that enrolled 140 patients with ostial renal artery stenosis greater than 50%, blood pressure controlled to less than 140/90 mmHg, and creatinine clearance 15 to 80 mL/min7). All patients received angiotensin blocking agents and a statin, regardless of their lipid levels. After a 2-year interventional period, no difference was observed in the decline of renal function, the degree of blood pressure control, and the rates of cardiovascular morbidity and death. Investigators in the ASTRAL trial enrolled 806 patients with at least one stenotic renal artery considered suitable for balloon angioplasty, stenting, or both in their international, multicenter trial8). The mean estimated GFR was 40 mL/min, and most of the patients were on statin and angiotensin blocking therapy. At a mean follow-up of 33.6 months, no difference was noted between the treatment groups in the decline of renal function or blood pressure control, and the renal function worsened slightly in both groups. Most of the enrolled patients in the two clinical trials had relatively asymptomatic atherosclerotic renal artery stenosis. Therefore, the practice of indiscriminately performing revascularization, without strong evidence, is no longer acceptable. The CORAL trial is an ongoing multicenter randomized controlled trial in the United States22). It is still enrolling patients that have drug-resistant hypertension or a GFR < 60 mL/min. It is using a standardized medical protocol to control blood pressure, and embolic protection devices during procedures are encouraged.
Thus far, intervention has not been recommended if renal function has remained stable over the past 6 to 12 months and if hypertension can be controlled medically. According to a clinical classification of atherosclerotic renal artery stenosis with guidelines for vascular intervention of surveillance published in 2008 by the Atherosclerotic Peripheral Vascular Symposium23), additional recommendations favoring medical therapy were as follows: very advanced age and/or limited life expectancy, extensive co-morbidities that make revascularization too risky, high risk for or previous experience with atheroembolic disease, and other concomitant renal parenchymal diseases that cause progressive renal dysfunction (e.g., interstitial nephritis, diabetic nephropathy)23). They also recommended factors favoring medical therapy and intervention as follows: progressive decline in GFR during treatment of systemic hypertension, failure to achieve adequate blood pressure control with optimal medical therapy (medical failure), rapid or recurrent decline in the GFR in association with a reduction in systemic pressure, decline in the GFR during therapy with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and recurrent congestive heart failure in a patient in whom the adequacy of left ventricular function does not provide an explanation23). The best evidence supporting intervention is for bilateral stenosis with "flash" pulmonary edema, but the evidence is from retrospective studies24-26).
The aim of treatment for ARVD should include the prevention of cardiovascular and/or renal events. Treatment of patients with ARVD should include consideration of the following factors: age, co-morbidity, blood pressure, renal function, and kidney size. The results of two recent studies suggest that patients with ARVD and stable renal function can be controlled medically. Intervention should be considered only in patients with ARVD and rapidly progressive cardiac or renal dysfunction. However, these conditions also increase the risk of complications related to revascularization procedures. Both the risks and potential long-term benefits of intervention should be considered. The results of the CORAL and the post hoc analysis of ASTRAL might provide additional evidence for revascularization.
References
1. Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002; 36:443–451. PMID: 12218965.

2. Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001; 60:1490–1497. PMID: 11576364.

3. Galaria II, Surowiec SM, Rhodes JM, et al. Percutaneous and open renal revascularizations have equivalent long-term functional outcomes. Ann Vasc Surg. 2005; 19:218–228. PMID: 15735947.

4. Kennedy DJ, Colyer WR, Brewster PS, et al. Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis. 2003; 42:926–935. PMID: 14582036.

5. Beutler JJ, Van Ampting JM, Van De Ven PJ, et al. Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol. 2001; 12:1475–1481. PMID: 11423576.
6. van de Ven PJ, Kaatee R, Beutler JJ, et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet. 1999; 353:282–286. PMID: 9929021.

7. Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009; 150:840–848. PMID: 19414832.
8. Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009; 361:1953–1962. PMID: 19907042.

9. Axelrod DA, Fendrick AM, Carlos RC, et al. Percutaneous stenting of incidental unilateral renal artery stenosis: decision analysis of costs and benefits. J Endovasc Ther. 2003; 10:546–556. PMID: 12932167.

10. Caps MT, Perissinotto C, Zierler RE, et al. Prospective study of atherosclerotic disease progression in the renal artery. Circulation. 1998; 98:2866–2872. PMID: 9860789.

11. Cheung CM, Patel A, Shaheen N, et al. The effects of statins on the progression of atherosclerotic renovascular disease. Nephron Clin Pract. 2007; 107:C35–C42. PMID: 17713349.

12. Pearce JD, Craven BL, Craven TE, et al. Progression of atherosclerotic renovascular disease: A prospective population-based study. J Vasc Surg. 2006; 44:955–962. discussion 962-953. PMID: 16982169.

13. Textor SC. Atherosclerotic renal artery stenosis: how big is the problem, and what happens if nothing is done? J Hypertens Suppl. 2005; 23:S5–S13. PMID: 16251847.

14. Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension. 2005; 45:1042–1049. PMID: 15897370.

15. Meier P, Rossert J, Plouin PF, Burnier M. Atherosclerotic renovascular disease: beyond the renal artery stenosis. Nephrol Dial Transplant. 2007; 22:1002–1006. PMID: 17210599.

16. Wright JR, Shurrab AE, Cheung C, et al. A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis. 2002; 39:1153–1161. PMID: 12046025.

17. Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001; 344:410–417. PMID: 11172177.

18. Chrysochou C, Cheung CM, Durow M, et al. Proteinuria as a predictor of renal functional outcome after revascularization in atherosclerotic renovascular disease (ARVD). QJM. 2009; 102:283–288. PMID: 19202165.

19. Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med. 2010; 77:178–189. PMID: 20200168.

20. Hiramoto J, Hansen KJ, Pan XM, Edwards MS, Sawhney R, Rapp JH. Atheroemboli during renal artery angioplasty: an ex vivo study. J Vasc Surg. 2005; 41:1026–1030. PMID: 15944605.

21. Hackam DG, Duong-Hua ML, Mamdani M, et al. Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J. 2008; 156:549–555. PMID: 18760140.

22. Cooper CJ, Murphy TP, Matsumoto A, et al. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J. 2006; 152:59–66. PMID: 16824832.

23. Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al. Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation. 2008; 118:2873–2878. PMID: 19106410.
24. Pickering TG, Herman L, Devereux RB, et al. Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet. 1988; 2:551–552. PMID: 2900930.

25. Messina LM, Zelenock GB, Yao KA, Stanley JC. Renal revascularization for recurrent pulmonary edema in patients with poorly controlled hypertension and renal insufficiency: a distinct subgroup of patients with arteriosclerotic renal artery occlusive disease. J Vasc Surg. 1992; 15:73–80. discussion 80-82. PMID: 1728693.

26. Bloch MJ, Trost DW, Pickering TG, Sos TA, August P. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens. 1999; 12:1–7. PMID: 10075377.

Fig. 1
Mechanisms of Renal Damage Associated with Atherosclerotic Renal Artery Stenosis. These mechanisms include an interaction among increased oxidative stress, immune responses, inflammation, immune responses, and angiotensin II/endothelin, which impair renal function, induce endothelial and epithelial dysfunction and injury, and may lead to irreversible scarring. Ox LDL, oxidized low-density lipoprotein cholesterol.
*Modified from the study of Chade AR et al. Ref. 14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download