Abstract
Background and purpose
At least 100 Ryanodine receptor type 1 (RYR1) mutations associated with malignant hyperthermia (MH) and central core disease (CCD) have been identified, but 2 RYR1 mutations accompanying multiminicore myopathy in an MH and/or CCD family have been reported only rarely.
Methods
Fifty-three members of a large MH family were investigated with clinical, histopathologic, RYR1 mutation, and haplotyping studies. Blood creatine kinase (CK) and myoglobin levels were also measured where possible.
Results
Sequencing of the entire RYR1 coding region identified a double RYR1 mutation (R2435H and A4295V) in MH/CCD regions 2 and 3. Haplotyping analysis revealed that the two missense heterozygous mutations (c.7304G>A and c.12891C>T) were always present on a common haplotype allele, and were closely cosegregated with histological multiminicores and elevated serum CK. All the subjects with the double mutation showed elevated serum CK and myoglobin, and the obtained muscle biopsy samples showed multiminicore lesions, but only two family members presented a late-onset, slowly progressive myopathy.
Conclusions
We found multiminicore myopathy with clinical and histological variability in a large MH family with an unusual double RYR1 mutation, including a typical CCD-causing known mutant. These results suggest that multiminicore lesions are associated with the presence of more than two mutations in the RYR1 gene.
Malignant hyperthermia (MH) is a genetically heterogeneous pharmacogenetic disorder of skeletal muscle that predisposes susceptible individuals to a high risk of life-threatening hypermetabolic episodes during anesthesia. It is triggered by commonly used inhaled anesthetics and/or depolarizing muscle relaxants, which lead to a rapid and sustained increase in myoplasmic Ca2+ from the sarcoplasmic reticulum in skeletal muscle. This induces skeletal-muscle rigidity, metabolic acidosis, cardiac arrhythmia, and high-fever reactions and, if not treated promptly, a possible fatal outcome.1,2
Central core disease (CCD) is a rare congenital myopathy with nonprogressive presentation that is closely associated with MH susceptibility (MHS). The diagnosis is mainly based on histological characteristics of cores, which are devoid of oxidative enzyme activity and predominantly observed in type I fibers. Typical clinical features include hypotonia, pronounced proximal muscle weakness, and skeletal anomalies such as congenital hip displacement and scoliosis. However, there are recent reports of a broader clinical and histological spectrum of CCD even within the same family, suggesting that using pathology alone can result in an incorrect diagnosis.3-5
Despite possessing six genetic loci and allelic heterogeneity implicated in MH,6,7 the ryanodine receptor type 1 (RYR1) gene is a primary locus for MHS [Online Mendelian Inheritance in Man (OMIM) 145600] and CCD (OMIM 117000).8-10 The RYR1 gene comprises 106 exons, encompasses about 160 kb of genomic DNA, maps to chromosome 19q13.1, and encodes a protein of 5,038 amino acids. The length and complexity of the RYR1 gene makes comprehensive screening for detecting mutation in the RYR1 gene difficult and laborious. 11-13 Most of the detected mutations linked to MH and CCD are concentrated in three defined regions of the RYR1 gene: between amino acids 35 and 614 (MH/CCD region 1), 2,129 and 2,458 (MH/CCD region 2) and 4,214 and 4,914 (MH/ CCD region 3);14 these have formed the main foci of mutation screening.
Recent analyses of mutations of the entire RYR1 coding region have increased the rate of mutation detection and involved searching many novel sequence variants outside the three mutational hot spots,15,16 with most being reported as point missense mutations.
Here we report for the first time the presence of an unusual double RYR1 mutation (Arg2435His and Ala4295Val) in a large Korean MH family with multiminicore myopathy. The results illustrate the cosegregation of the two compound mutations with multiminicore myopathy and suggest that significant clinical and histological phenotypic variability is associated with the present novel mutations.
As shown in Fig. 1, 53 members of the family were included in this study. All blood and tissue samples were obtained from the 53 consenting family members with ethical approval from the ethics committee of Chonbuk National Hospital. Serum creatine kinase (CK) and myoglobin levels were measured in 24 subjects and muscle biopsies were performed in 9 subjects (Table 1).
The investigation of the family started after the MH attack of proband III-17. He underwent general anesthesia with thiopental sodium, succinylcholine and enflurane for orthopedic surgery. About 30 min after anesthesia, he developed hyperthermia (40℃), tachycardia (180 beats/min) and generalized muscle rigidity, accompanied by dark-colored blood in the surgical field and cola-colored urine. His serum myoglobin and CK levels were >500 ng/mL and 25,000 IU/L, respectively. He was treated with dantrolene sodium (2 mg/kg) and recovered without any sequelae. According to the clinical grading scale,17 the MH raw score was calculated as 78, and this episode could be classified as Group 6, almost certainly corresponding to MH. Individual III-4 died when she was 20 years old due to an MH attack during the correction of congenital ptosis under succinylcholine and halothane.18 Individual III-9, a 9-year-old female, developed an MH attack during general anesthesia for appendectomy, but was treated with dantrolene sodium and recovered completely.
Only two of the family members, individuals II-1 and III-7, showed clinically evident myopathy. They were father and son, and presented late-onset chronic progressive muscle weakness and atrophy of the hip girdle and the proximal portion of the lower legs. A thorough history-taking from the father revealed that mild muscle weakness of the lower limbs first appeared at the age of 30 years, with the subsequent slow progression of muscle wasting. Finally, at 65 years of age, he showed marked muscle atrophy that meant that he could not stand or walk without a support (MRC grade IV-). The son was 27 years old when a physical examination was performed by a neurologist (Sunwoo IN) and he complained of muscle weakness of the lower limbs since he was 24 years old. Muscle weakness and wasting of the lower limbs subsequently gradually progressed over 10 years, and he found it very difficult to stand or to climb stairs.
Genomic DNA was extracted from whole blood samples using the GENErALL™ nucleic acid purification kit (General Biosystem, Korea) according to the manufacturer's instructions. Mutation analyses for two MH probands (III-9 and III-17) and two myopathic patients (II-1 and III-7) were performed from the entire coding region of the RYR1 gene by a polymerase chain reaction (PCR) concatenation and sequencing method, as described previously.19 Briefly, all PCR primer pairs for amplification were designed in the appropriate regions of intron sequences from genomic DNA (all of the primer sequences are available on request). Complementary 5' tails for 12-bp overlapping concatenation arms were added to the primers to form concatemers. The second round of PCR for the concatenation reaction was performed using the first-round PCR product as the template. The concatenated PCR products were purified using the QIAquick PCR purification kit (QIAGEN, USA) for sequencing. The purified concatemers were sequenced in both directions using BigDye Terminator chemistry on an automatic DNA sequencer (Model 3700, ABI PRISM, USA). The sizes of the first PCR products and each concatemer for sequencing ranged from 500 to 700 bp. The total number of PCR concatemers for bidirectional sequencing was 47, comprising 16 first-round PCR products for direct sequencing, and 26 and 5 concatemers consisting of 2 and 3 first-round PCR products, respectively.
HgaI restriction enzyme analysis was performed for screening the R2435H mutation, as described previously.20 For screening the A4295V mutation using direct sequencing, a 598-bp product in exon 91 of RYR1 was amplified by PCR using forward (5'-tgtagctgccactgcgctgtcg-3') and reverse (5'-tgccaggagctcggtcac-3') primers in a 50-µL reaction mixture with 200-500 ng of gDNA, each primer at 0.2 µM, each dNTP at 2.5 mM, 2 mM MgCl2, 50 mM KCl and 2.5 U of TAKARA LA Taq™ with GC buffer (TAKARA BIO, Japan). The reaction consisted of an initial 1-min denaturation at 94℃, followed by 30 cycles of 94℃ for 30 sec, 60℃ for 30 sec and 72℃ for 2 min. The PCR product was purified and directly sequenced in both directions. The two mutations were also screened in samples from 100 normal controls.
The D19S191, D19S422, D19S220, D19S190 and D19S223 chromosome-19 microsatellite markers flanking the RYR1 locus from members of the family, as described previously,21 were genotyped using a DNA analyzer (ABI 3100) in conjunction with GENESCAN and GENOTYPER software (Applied Biosystems, Foster City, CA).
Muscle specimens were obtained from the vastus lateralis or biceps brachii under local anesthesia in nine individuals harboring the R2435H and A4295V mutations. Specimens were frozen immediately in isopentane-cooled liquid nitrogen and stored at -80℃ until processed for histochemistry studies. Sections of fresh-frozen muscle were stained for hematoxylin and eosin, adenosine triphosphatase (ATPase), periodic acid-Schiff, Sudan Black and nicotinamide adenine dinucleotide dehydrogenase-tetrazolium reductase (NADH-TR). Specimens for electron microscopy were fixed in 5% glutaraldehyde and 1% osmium tetraoxide in 0.1 M cacodylate buffer. Histochemistry and electron microscopy samples were processed as described previously.22
The presented pedigree contained two probands (III-9 and III-17) who survived MH episodes, one individual (III-4) who died from an MH episode, and two patients (II-1 and III-7) showing clinically definite myopathy.
A double mutation (Arg2435His and Ala4295Val) was simultaneously identified in the family from scanning mutations of the entire RYR1 coding region. A known c.7304G>A mutation in exon 45 was responsible for the substitution of an arginine by a histidine residue at position 2,435 of RYR1 (R2435H). A novel c.12891C>T mutation in exon 91 led to the substitution of a conserved alanine by a valine residue at position 4295 (A4295V). These two mutations were missense and heterozygous (Fig. 1). Twenty-eight family members with the R2435H mutation (Fig. 2) were diagnosed as having MHS without performing the invasive in vitro contracture test according to guidelines of the European Malignant Hyperthermia Group (EMHG).23
Neither of the two mutations were detected in the 100 normal controls. Additionally, 20 silent polymorphisms with heterozygous or homozygous changes were identified throughout the RYR1 gene, 7 of which were identified for the first time, as presented in Table 2.
The two R2435H and A4295V mutations were screened by HgaI enzyme digestion20 and a direct sequencing method, respectively. Twenty-eight of the 53 family members were compound heterozygous individuals harboring the two RYR1 mutations (Fig. 2, Table 1). Haplotyping analysis showed that both mutations were always present in the 4-6-8-3-7 common haplotype for the D19S191, D19S220, D19S422, D19S190 and D19S223 markers, and cosegregated with multiminicore lesions in muscle specimens, and showed variably elevated serum CK and myoglobin (Fig. 2, Table 1).
Histochemistry revealed variation in fiber size with increasing internal nuclei, and multiminicore structures with scattered moth-eaten appearances and unclear border margins. There was no type I fiber predominance, which is known to be one of pathognomonic findings of CCD (Fig. 3). The electron microscopy examination of the samples revealed similar minicore-like structures with a mean cross-sectional diameter of 8-12 µm with streaming or disruption of Z-lines (Fig. 4).
The present study is the first showing a double RYR1 mutation (Arg2435His and Ala4295Val) in a MH family with features of multiminicore myopathy, which differs from typical CCD with the R2435H mutation only. Members of this family with the double mutation showed elevated serum CK and myoglobin, and the obtained muscle biopsy samples showed multiminicore lesions, but only two members represented a late-onset, slowly progressive myopathy.
Most RYR1 mutations associated with MH and CCD have been reported as a private mutation having a single missense mutation in a family.14,24 However, the family in the present study showed two amino acid substitutions in the 4-6-8-3-7 common haplotype of the RYR1 gene (Fig. 1), which was completely inherited as an autosomal dominant trait over three generations (Fig. 2). Therefore, this family differs from a few reported MH cases having two RYR1 mutants located on each haplotype arising from their affected parent.21,25,26 Only one previously report described the presence of two RYR1 mutants (R2676W and T2787S) in the same copy of the RYR1 gene in an MHS family.27
In addition to the R2435H mutation, which was reported previously20,28 and included in the list of 22 (EMHG) and 17 (North American Malignant Hyperthermia Group) RYR1 mutations for MHS and CCD,23,29 the combined A4295V substitution might play an important role in the variable myopathic phenotypes. However, it was difficult to assess the pathogenic role of A4295V substitution in this family due to the uniform presence of the two mutations on the same allele. There are several reasons for considering the A4295V substitution a candidate mutation for MHS and/or multiminicore myopathy: 1) it segregated with the multiminicore lesions and elevated blood CK and myoglobin; 2) it was located in the mutational hot spot of MH/CCD region 3 and relatively close to the V4234L mutation, which is known to cause MHS;30 3) the alanine at amino acid position 4,295 is conserved in vertebrates (Fig. 5); 4) it was not found in the family members without the common haplotype or in the 100 unrelated healthy controls; and 5) the A4295V missense substitution occurred in a CpG dinucleotide, which is consistent with the observation that most mutations of the RYR1 gene (but not polymorphisms) occur in CpG dinucleotide sequences, presumably through fixing of T : G mismatch premutations arising from deamination of 5-methyl-cytidine residues.31,32 However, the extent to which the A4295V substitution contributes to the pathogenic effects requires further investigation.
A study showing a high occurrence of multiminicore lesions in the MHS family found two mutations of R2676W and T2787S throughout the RYR1 cDNA.27 Because the T2787S substitution was identified in another MHS family, those authors suggested that the second R2676W substitution is responsible for the cores. Also, there are a few reports of segregation between the presence of two RYR1 mutations and the cores or multiminicores in one family.33,34 In the same way, the presence of the A4295V mutation in addition to the known R2435H mutation might have been responsible for the multiminicore lesions in the present family. Also, our results suggest that the multiminicore or core lesions are associated with more than two RYR1 mutations.
Clinical and histopathological overlap among different congenital myopathies could be responsible for the diagnostic dilemma. Therefore, identifying the genetic determinants might aid a definitive diagnosis.34 The identification of the known R2435H mutation in the present family strongly suggested that CCD was present. The typical symptoms of CCD comprise congenital hypotonia, nonprogressive proximal muscle weakness, and myopathic facies.35 However, the clinical features in the present family were quite different and more variable. Among the 28 family members with the 2 mutations (Fig. 2), 2 subjects (II-1 and III-7) showed an overt clinical myopathy with a late-onset and slow progression, while there were no clinical myopathic symptoms among the other family members despite the presence of elevated serum CK and myoglobin. On the contrary, some individuals (particularly I-9 and II-13) had a burly physique despite having both multiminicore lesions and markedly elevated serum CK and myoglobin. In addition to the variable phenotypes from the same mutations, the muscle specimens also showed inconsistent findings. The son (III-7) showed no obvious core structures with the oxidative enzyme stain, whereas his father (II-1) exhibited numerous multiminicores. This finding is consistent with the core structures being a secondary phenomenon that appears with aging or disease progression.4,36 Overall, the clinical and histological features in the present family raise the possibility of a chronic progressive myopathy with multiminicores in which clinical severity is not correlated well with the pathologic findings and the levels of blood CK and myoglobin.
To date, the complexity of the RYR1 gene has meant that the detection of mutations therein has focused on three mutational hot spots. Recent mutation analysis of the entire RYR1 coding region by the DHPLC method and sequencing of full-length leukocyte RYR1 cDNA has increased the rate of detection of mutations in up to 70% of affected MH families.15,37 In the present study, we used PCR concatenation followed by sequencing, which reduced the PCR fragments for sequencing from 106 to 47 per patient. We consider that this could be a clinically useful genetic test for scanning mutations of the entire RYR1 coding region, considering that sequencing is a final confirmatory step for DNA-based diagnosis. Additionally, 20 silent polymorphisms including 7 novel ones were detected in the RYR1 coding region. Therefore, scanning of the entire RYR1 coding region should be carefully considered, especially in cases with variable or unusual clinical and pathologic phenotypes.
In conclusion, we found a double RYR1 mutation (Arg2435His and Ala4295Val) in a MH family with multiminicore myopathy. The two amino acid substitutions were found in the common haplotype of the RYR1 gene with an autosomal dominant trait. In addition to the Arg2435His mutation, which has previously been shown to cause CCD, the novel Ala4295Val substitution is a candidate mutation responsible for multiminicore myopathy.
Figures and Tables
Fig. 1
Pedigree and haplotyping analysis of the present family. Arrows denote probands who experienced malignant hyperthermia episodes or individuals showing overt clinical myopathies. Black shapes indicate individuals with histological multiminicore lesions. Empty shapes and a question mark denote individuals in whom muscle biopsies and genetic tests were not performed, respectively. Genotypes: +/-, heterozygous carrier of R2435H and A4295V mutations; -/-, no mutation. The results of marker typing and identification of the mutation are shown in the following order: D19S191-D19S220-RYR1 mutations-D19S422-D19S190-D19S223.
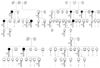
Fig. 2
DNA sequencing of the regions of the ryanodine receptor type 1 (RYR1) gene in which the 7304CGC>CAC (Arg 2435His) missense mutation in exon 45 and 12891GCG>GTG (Ala4295Val) missense mutation in exon 91 were detected. Individual III-3 without the two mutations showed a single peak, but individual III-17 (a proband with an MH episode) showed two superimposed peaks (arrows) in both exons 45 and 91, indicating heterozygous missense mutations. F and R, forward and reverse sequencing, respectively.

Fig. 3
Muscle biopsy sections stained for NADH-TR with a scattered motheaten appearance and unstained multiminicores in the father (II-1, A) and subtle moth-eaten changes in his son (III-7, B).
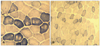
Fig. 4
Electron micrographs showing streaming and disruption of irregular Z-lines, and the amorphous cores from individuals III-17 (A) and III-1 (B).
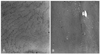
Fig. 5
Amino acid sequence alignments in the region of RYR1 isoforms flanking the A4295V mutation in human, pig and rabbit. *Denotes the conserved segment in A4295V.

References
2. Hopkins PM. Malignant hyperthermia: advances in clinical management and diagnosis. Br J Anaesth. 2000. 85:118–128.

3. Quinlivan RM, Muller CR, Davis M, Laing NG, Evans GA, Dwyer J, et al. Central core disease: clinical, pathological, and genetic features. Arch Dis Child. 2003. 88:1051–1055.

4. Sewry CA, Müller C, Davis M, Dwyer JS, Dove J, Evans G, et al. The spectrum of pathology in central core disease. Neuromuscul Disord. 2002. 12:930–938.

5. Mertz KD, Jost B, Glatzel M, Min K. Progressive scoliosis in central core disease. Eur Spine J. 2005. 14:900–905.

6. Jurkat-Rott K, McCarthy T, Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve. 2000. 23:4–17.

7. Robinson RL, Curran JL, Ellis FR, Halsall PJ, Hall WJ, Hopkins PM, et al. Multiple interacting gene products may influence susceptibility to malignant hyperthermia. Ann Hum Genet. 2000. 64:307–320.

8. MacLennan DH, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk RG, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990. 343:559–561.

9. McCarthy TV, Healy JM, Heffron JJ, Lehane M, Deufel T, Lehmann-Horn F, et al. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature. 1990. 343:562–564.

10. Kausch K, Lehmann-Horn F, Janka M, Wieringa B, Grimm T, Müller CR. Evidence for linkage of the central core disease locus to the proximal long arm of human chromosome 19. Genomics. 1991. 10:765–769.

11. Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989. 339:439–445.

12. Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA, et al. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990. 265:2244–2256.

13. Phillips MS, Fujii J, Khanna VK, DeLeon S, Yokobata K, de Jong PJ, et al. The structural organization of the human skeletal muscle ryanodine receptor (RYR1) gene. Genomics. 1996. 34:24–41.

14. Brini M, Manni S, Pierobon N, Du GG, Sharma P, MacLennan DH, et al. Ca2+ signaling in HEK-293 and skeletal muscle cells expressing recombinant ryanodine receptors harboring malignant hyperthermia and central core disease mutations. J Biol Chem. 2005. 280:15380–15389.

15. Sambuughin N, Holley H, Muldoon S, Brandom BW, de Bantel AM, Tobin JR, et al. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the north american population. Anesthesiology. 2005. 102:515–521.

16. Brandom BW, Muldoon SM. XXIVth Annual Meeting of the European Malignant Hyperthermia Group. Anesthesiology. 2005. 103:1324.

17. Larach MG, Localio AR, Allen GC, Denborough MA, Ellis FR, Gronert GA, et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology. 1994. 80:771–779.

18. Kim JS, Moon JI, Lee JH. Malignant hyperthermia during general anesthesia (a case report). Korean J Anesthesiol. 1981. 14:313–318.

19. Higasa K, Hayashi K. Ordered catenation of sequence-tagged sites and multiplexed SNP genotyping by sequencing. Nucleic Acids Res. 2002. 30:E11.

20. Zhang Y, Chen HS, Khanna VK, De Leon S, Phillips MS, Schappert K, et al. A mutation in the human ryanodine receptor gene associated with central core disease. Nat Genet. 1993. 5:46–50.

21. Monnier N, Krivosic-Horber R, Payen JF, Kozak-Ribbens G, Nivoche Y, Adnet P, et al. Presence of two different genetic traits in malignant hyperthermia families: implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology. 2002. 97:1067–1074.

22. Romero NB, Nivoche Y, Lunardi J, Bruneau B, Cheval MA, Hillaire D, et al. Malignant hyperthermia and central core disease: analysis of two families with heterogeneous clinical expression. Neuromuscul Disord. 1993. 3:547–551.

23. Urwyler A, Deufel T, McCarthy T, West S; European Malignant Hyperthermia Group. Guidelines for molecular genetic detection of susceptibility to malignant hyperthermia. Br J Anaesth. 2001. 86:283–287.

24. Treves S, Anderson AA, Ducreux S, Divet A, Bleunven C, Grasso C, et al. Ryanodine receptor 1 mutations, dysregulation of calcium homeostasis and neuromuscular disorders. Neuromuscul Disord. 2005. 15:577–587.

25. Lynch PJ, Krivosic-Horber R, Reyford H, Monnier N, Quane K, Adnet P, et al. Identification of heterozygous and homozygous individuals with the novel RYR1 mutation Cys35Arg in a large kindred. Anesthesiology. 1997. 86:620–626.

26. Rueffert H, Olthoff D, Deutrich C, Thamm B, Froster UG. Homozygous and heterozygous Arg614Cys mutations (1840C-->T) in the ryanodine receptor gene co-segregate with malignant hyperthermia susceptibility in a German family. Br J Anaesth. 2001. 87:240–245.

27. Guis S, Figarella-Branger D, Monnier N, Bendahan D, Kozak-Ribbens G, Mattei JP, et al. Multiminicore disease in a family susceptible to malignant hyperthermia: histology, in vitro contracture tests, and genetic characterization. Arch Neurol. 2004. 61:106–113.

28. Shuaib A, Paasuke RT, Brownell KW. Central core disease. Clinical features in 13 patients. Medicine (Baltimore). 1987. 66:389–396.
29. Sei Y, Sambuughin N, Muldoon S. Malignant hyperthermia genetic testing in North America Working Group Meeting. Bethesda, Maryland. September 4-5, 2002. Anesthesiology. 2004. 100:464–465.

30. Gillard EF, Otsu K, Fujii J, Duff C, de Leon S, Khanna VK, et al. Polymorphisms and deduced amino acid substitutions in the coding sequence of the ryanodine receptor (RYR1) gene in individuals with malignant hyperthermia. Genomics. 1992. 13:1247–1254.

31. McCarthy TV, Quane KA, Lynch PJ. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum Mutat. 2000. 15:410–417.

32. Tilgen N, Zorzato F, Halliger-Keller B, Muntoni F, Sewry C, Palmucci LM, et al. Identification of four novel mutations in the C-terminal membrane spanning domain of the ryanodine receptor 1: association with central core disease and alteration of calcium homeostasis. Hum Mol Genet. 2001. 10:2879–2887.

33. Monnier N, Kozak-Ribbens G, Krivosic-Horber R, Nivoche Y, Qi D, Kraev N, et al. Correlations between genotype and pharmacological, histological, functional, and clinical phenotypes in malignant hyperthermia susceptibility. Hum Mutat. 2005. 26:413–425.

34. Shepherd S, Ellis F, Halsall J, Hopkins P, Robinson R. RYR1 mutations in UK central core disease patients: more than just the C-terminal transmembrane region of the RYR1 gene. J Med Genet. 2004. 41:e33.

35. Mathews KD, Moore SA. Multiminicore myopathy, central core disease, malignant hyperthermia susceptibility, and RYR1 mutations: one disease with many faces? Arch Neurol. 2004. 61:27–29.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download