Abstract
Neurological disorders induced by long-term exposure to organic solvents typically have a slowly progressive clinical course, which may be arrested or even reversed following discontinuation of exposure. We report an unusual case of rapidly progressive toxic leukoencephalomyelopathy in a 29-year-old man who had worked at a chemical factory that used toluene for the manufacture of nylon 66 for 5 years. He presented with progressive weakness of legs, recurrent seizures, and cognitive decline. Widespread white-matter changes in the brain and spinal cord, and myelodysplastic syndrome were noted. He died 6 months after the onset of his symptoms, and autopsy showed discrete multifocal demyelination and necrosis in the central nervous system, and dysplastic cells of erythroid, myeloid, and megakaryotic lineages in blood vessels. The co-occurrence of leukoencephalomyelopathy and myelodysplastic syndrome highlights the vulnerability of the white matter and bone marrow to injury from organic solvents. Intravascular congestion of dysplastic hematopoietic cells might have led to his unusually rapid progression of leukoencephalomyelopathy.
Organic solvents are used in various occupational settings, and brain damage resulting from long-term exposure to organic solvents is a health concern to many workers. There are numerous clinical reports1-10 and experimental studies11,12 of organic solvent-induced disorders involving the central nervous system (CNS), peripheral nervous system (PNS), and autonomic nervous system, and bone marrow and other organs. Neurological disorders induced by toxins typically run a monophasic course. Although progression may occur for several weeks after discontinuation of exposure, it is eventually arrested, after which improvement may follow.1
Here we describe an unusual case of rapidly progressive leukoencephalomyelopathy coexisting with myelodysplastic syndrome (MDS) following chronic exposure to organic solvents, and discuss the pathogenetic interconnection between these conditions.
A 29-year-old man was admitted due to progressive weakness of both legs over the previous 4 months, and cognitive decline and recurrent seizures over the previous month. The weakness had started in his right leg and gradually spread to the left side over 1 month. Cranial and spinal magnetic resonance imaging (MRI) performed at another institution 3 months prior to admission to our hospital showed multifocal high-intensity signals in the white matter on T2-weighted imaging (Fig. 1A-C). Cerebrospinal fluid (CSF) parameters including the IgG index and oligoclonal band were normal. The score on the Korean version of the Mini-Mental State Examination (K-MMSE) was 27/30 (education length: 15 years). Intravenous methylprednisolone treatment was given, but the weakness worsened, and generalized tonic-clonic seizures occurred 1 month prior to admission. He was transferred to another hospital. Repeated application of the K-MMSE showed a severe decline in cognitive function (9/30), and abdominal distension was detected 10 days prior to admission. Abdominal computed tomography revealed hepatosplenomegaly, ascites, and pleural effusions. The ascitic fluid was transudate. He was then transferred to our hospital.
He had no specific history of medical or neurological disease, and took no illicit drugs. A family history was absent, but his occupational history was notable. He had worked at a chemical factory manufacturing nylon 66 for 5 years, resulting in exposure to toluene. However, no information was available on the degree of exposure of either the patient or his coexposed workers. A neurological examination revealed severe apathy, disorientation to time and place, dementia (K-MMSE: 9/30), decreased visual sensitivity in both eyes but with normal papillary reflex, flaccid paraplegia with no sensory perception below level T4 of the spinal cord, and bilateral extensor toe sign.
Routine blood tests were normal except for mild leukocytosis (11,000 cells/mm3). Rheumatoid factor, anti-Ro/La, anti-Sm, antimitochondrial, antinuclear, and anti-double-stranded DNA antibodies, HIV test, very-long-chain fatty acid, arylsulfatase A, beta-galactosidase, and CSF profiles were normal. His visual evoked potential revealed the presence of bilateral retrochiasmal lesions. Cranial T2-weighted MRI showed more extensive and symmetric high-intensity signals in the periventricular white matter than in his previous MRI scans, and symmetric hypointensities in the thalamus (Fig. 1-D, F). Spinal T2-weighted MRI also revealed extensive high-intensity signals throughout the spine with mild atrophy. Electromyography and nerve conduction studies were normal (Fig. 1-G, H).
He became febrile (39℃) on his 6th hospital day (HD). Anemia (Hb: 9.1 g/dl) and thrombocytopenia (79,000 platelets/mm3) were detected. A peripheral-blood morphology examination showed macrocytic, normo chromic red blood cells with anisocytosis and polychromasia. Ascites, hepatomegaly, and pleural effusions worsened. A needle liver biopsy performed on the 10th HD showed nonspecific reactive hepatitis. Despite transfusion, the thrombocytopenia and anemia worsened (Hb: 8.3 g/dl; 58,000 platelets/mm3), and his cognitive functions deteriorated. Recurrent generalized tonic seizures occurred after the administration of multiple antiepileptic drugs. On the 30th HD he did not respond to visual threatening despite his pupils being reactive. He was abulic and akinetic. On the 55th HD he died of uncontrolled pneumonia, and a postmortem examination was performed.
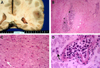
The unfixed cerebrum and cerebellum weighed 1,165 and 390 g, respectively. The meninges were not opacified. Coronal sections (Fig. 2-A) showed bilateral, discrete, and friable gray-yellow discolored foci in the white matter that was accompanied by punctate hemorrhage. The brain stem was grossly unremarkable, but multiple, discrete, and confluent foci of necrosis and demyelination were observed microscopically (Fig. 2-B, D) that were randomly scattered in the subcortical and deep white matter of the cerebrum, cerebellum, brain-stem, and spinal cord. These foci were associated with collections of foamy histiocytes and dystrophic calcification. The adjacent, relatively uninvolved parenchyma showed patchy spongiform changes and reactive astrocytosis. Blood vessels were filled with dysplastic cells of erythroid, myeloid, and megakaryotic lineages (Fig. 2-D). The bone marrow was hypercellular (91-100%), and showed a proliferation of erythropoietic cells, granulopoietic cells, and megakaryocytes with dysplastic features, which was consistent with trilineage MDS. Heavy infiltrates of dysplastic hematopoietic cells were evident in multiple organs, including the lung, heart, kidney, liver, and spleen. Organizing pneumonia with cytomegalovirus infection was noted in both lungs.
Toxic leukoencephalopathy is an important differential diagnosis in any patient who presents with acute or chronic neurobehavioral deficits and who has a possible or known exposure to organic solvents.13 In our case, the history of chronic occupational exposure, systemic involvement such as MDS and hepatitis, and the exclusion of other possible causes support the diagnosis of toxic leukoencephalopathy.
Toluene, the main constituent of glue, paint, and thinner, is widely recognized as an inducer of leukoencephalopathy.4 Inhaled toluene is rapidly absorbed by the lungs and excreted as a form of hippuric acid by the kidney.13 Because of its high lipid solubility, it easily enters highly vascularized lipid-rich tissues such as the brain and the bone marrow.4,13 Therefore, toluene easily damages these tissues, resulting in demyelination, gliosis, axonal loss, and iron deposition in the nervous system and the malignant transformation of blood-forming tissue in the bone marrow. Many reports on toluene abusers have established that toluene caninduce various neurological disorders, such as encephalopathy, cerebellar and pyramidal signs, and peripheral neuropathy.1-5 Clinical observations have also demonstrated that exposure to organic solvents can cause acute myeloid leukemia or MDS.6-10
Neurotoxicity disorders should be suspected when there is a close relationship between clinical onset and prior exposure to a chemical agent, especially one known to be neurotoxic. However, inaccurate information on the degree and duration of exposure, ill-defined dose-response relation, litigation issues, and limitations of diagnostic methods hamper the diagnosis of toxic leukoencephalopathy.1,13 The usefulness of laboratory testing is limited by the exposure often occurring years previously and no test existing to identify the suspected toxin.1 The reported MRI findings of chronic solvent abusers are cerebral and cerebellar atrophy, multifocal or diffuse white-matter changes, loss of demarcation between the cortex and white matter, and symmetric hypointensities in the thalamus or basal ganglia.14 However, the radiological findings are not sufficiently specific to allow a conclusive diagnosis. Furthermore, pathological studies on solvent-induced leukoencephalopathy are very rare.
Kornfeld, et al. first reported the pathology of this disorder in three cases.15 They described patchy demyelination in the white matter of the cerebral and cerebellar hemispheres that was associated with trilaminar inclusions within PAS-positive macrophages. A comparative study of multiple sclerosis and solvent-induced leukoencephalopathy produced similar pathological findings for the demyelination process.16 Experimental studies11,12 aimed at revealing causal relationships showed that high-dose exposure can result in multifocal axonal loss, gliosis, dystrophic calcification, and demyelination. These findings were also obtained in our case, which suggests the presence of aggressive pathogenesis.
Most cases of neurological disorders induced by organic solvents typically have a slow progression, whereas our patient showed a rapidly progressive disease course. We speculate that the coexistent MDS accelerated damage to the white matter. Remarkably, intracerebral vessels in the white matter were filled with dysplastic cells of erythroid, myeloid, and megakaryotic lineages. These dysplastic cells might have damaged the white matter by hyperviscosity leading to hemorheological disturbances and microvasculopathy.17
Another interesting point was also raised by this case. The PNS and CNS are potential targets for solvent-induced neurotoxicity.1 However, the PNS was not affected in this patient, and so the selective involvement of the CNS suggests that the PNS is more resistant to direct toxic injury and to ischemic damage that is attributed to hyperviscosity. The PNS might only become involved when the disease process is more gradual and chronic
To the best of our knowledge, this is the first reported case of rapidly progressive leukoencephalomyelopathy induced by chronic and occupational exposure to an organic solvent. The unique pathological findings support the presence of pathogenetic interconnections between rapidly progressive leukoencephalomyelopathy and MDS.
Figures and Tables
Figure 1
Cranial axial T2-weighted MRI images show multiple high-intensity signals in the periventricular white matter and symmetric hypointensities in the thalamus (A, C), and a T1-weighted MRI image shows multifocal calcification in the periventricular white matter (B). Follow-up brain MRI performed 3 months later (D-F) shows more extensive, bilateral, and symmetrical high-intensity signals in the white matter. Note that the differentiation between the gray and white matter is preserved. Spinal T1-weighted MRI image shows mild atrophy (G), and T2-weighted MRI reveals extensive high-intensity signals throughout the spine (H).
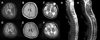
Figure 2
Coronal cross section of the brain shows diffuse gray-yellow discoloration and softening of the white matter (A). The white matter shows a diffuse area of necrosis (white arrowheads) with dystrophic calcification (black arrowheads; B), and the high-magnification view of the necrotic white matter shows rarefaction and occasional reactive astrocytes (white arrow; C). The cerebral blood vessel was filled with dysplastic hematopoietic cells. (D) Black arrow indicates a foam cell in the necrotic white matter.
References
1. Dryson EW, Ogden JA. Organic solvent induced chronic toxic encephalopathy: extent of recovery, and associated factors, following cessation of exposure. Neurotoxicology. 2000. 21:659–665.
2. Malm G, Lying-Tunell U. Cerebellar dysfunction related to toluene sniffing. Acta Neurol Scand. 1980. 62:188–190.

3. Hormes JT, Filley CM, Rosenberg NL. Neurologic sequelae of chronic solvent vapor abuse. Neurology. 1986. 36:698–702.

4. Rosenberg NL, Kleinschmidt-DeMasters BK, Davis KA, Dreisbach JN, Hormes JT, Filley CM. Toluene abuse causes diffuse central nervous system white matter changes. Ann Neurol. 1988. 23:611–614.

5. Filley CM, Heaton RK, Rosenberg NL. White matter dementia in chronic toluene abuse. Neurology. 1990. 40:532–534.

6. Brandt L. Exposure to organic solvents and risk of haematological malignancies. Leuk Res. 1992. 16:67–70.

7. Rinsky RA, Smith AB, Hornung R, Filloon TG, Young RJ, Okun AH, et al. Benzene and leukemia. An epidemiologic risk assessment. N Engl J Med. 1987. 316:1044–1050.

8. Lindquist R, Nilsson B, Eklund G, Gahrton G. Increased risk of developing acute leukemia after employment as a painter. Cancer. 1987. 60:1378–1384.

9. Rigolin GM, Cuneo A, Roberti MG, Bardi A, Bigoni R, Piva N, et al. Exposure to myelotoxic agents and myelodysplasia: case-control study and correlation with clinicobiological findings. Br J Haematol. 1998. 103:189–197.

11. Graham DI. Greenfield's neuropathology. 1997. 6th edition. Arnold;779–780.
12. Lees-Haley PR, Williams CW. Neurotoxicity of chronic low-dose exposure to organic solvents: a skeptical review. J Clin Psychol. 1997. 53:699–712.

14. Aydin K, Sencer S, Demir T, Ogel K, Tunaci A, Minareci O. Cranial MR findings in chronic toluene abuse by inhalation. Am J Neuroradiol. 2002. 23:1173–1179.
15. Kornfeld M, Moser AB, Moser HW, Kleinschmidt-DeMasters B, Nolte K, Phelps A. Solvent vapor abuse leukoencephalopathy. Comparison to adrenoleukodystrophy. J Neuropathol Exp Neurol. 1994. 53:389–398.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download